Thermodynamic potential
In thermodynamics, thermodynamic potentials are quantities whose information content fully describes the behavior of a thermodynamic system at equilibrium. The independent state variables of a thermodynamic potential are called its natural variables if their derivative of the potential is equal to one of the dependent state variables (for example: 


Thermodynamic potentials, which are energies, can be 

In addition, there are other thermodynamic potentials that are not energies, for example, the entropy 
The notion of thermodynamic potential was generalized by Gottfried Falk to the notion of Massieu-Gibbs functions (after Josiah Willard Gibbs), which are corresponding state functions in not necessarily thermodynamic systems.
Physical meaning
An extreme value (not always a minimum) of a thermodynamic potential indicates thermodynamic equilibrium.
Thus, after the connection of one closed system to another, a thermodynamic equilibrium has been established as soon as the entropy of the total system is maximal. In this case, all intensive parameters of the two systems are also equal in each case:
Moreover, thermodynamic potentials summarize the equations of state of the system, since these are accessible by differentiating a thermodynamic potential according to its dependent variables.
Description
The internal energy 

- From entropy
to temperature
, since ∂
- from volume
to pressure
, since ∂
- From particle number
to chemical potential μ
, since ∂
Given these 3 pairs of variables, there are 
| Thermodynamic potentials | ||||
| Name (alternative name) | Formula symbol | natural variables | Characteristic function f | Interrelationships |
| Inner energy | | | |
|
| Free energy | | | | |
| Enthalpy | | | | |
| Gibbs energy | | | | |
| - | | | | |
| Great Cannon Potential | | | | |
| - | | | | |
| - | |
| | |
All meaningful thermodynamic potentials arising from U(S,V,N) by Legendre transformation provide the same complete information about a system. However, the simplest description of the system provides only one of the potentials, depending on the ensemble; this is extremal at equilibrium.
One way to remember the thermodynamic potentials with their natural variables is the Guggenheim square.
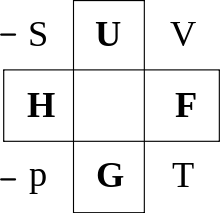
Guggenheim Square
Search within the encyclopedia


































