Steroid
Steroids (Greek στερεοειδές, from στερεός stereós, German 'solid' and the adjectival suffix -id, Latinization of Ancient Greek -ειδής -eidḗs "[similar to the noun]", from εἶδος eîdos "appearance, shape, kind") are a substance class of lipids (molecules with lipophilic groups, usually insoluble in water). Formally, steroids are derivatives of the hydrocarbon sterane (cyclopentanoperhydrophenanthrene). Steroids belong to the isoprenoids, more precisely to the triterpenoids.
Natural steroids occur in animals, plants and fungi; many are synthesized in the smooth endoplasmic reticulum. Their biochemical functions range from the production of vitamins and sex hormones (androgens in men and estrogens in women) to bile acid and toad toxins to the heart-active toxins of digitalis and oleander.
The name of the substance class is derived from the first known steroid, cholesterol. In animals and in the human organism, cholesterol is the most important steroid; plants, on the other hand, contain it only in small quantities. Cholesterol is the basis for lipoproteins and steroid hormones, e.g. the hormones of the adrenal cortex (corticosteroids). Artificial derivatives of the male sex hormone testosterone, which belongs to the steroids, the anabolic steroids, are used as muscle building preparations and are therefore also known as doping agents.
The total synthesis of steroids was first achieved in 1939 with equilenin and in 1948 with estrone, both aromatic steroids. In the case of non-aromatic steroids such as cholesterol and cortisone, the breakthrough was achieved independently in 1951 by the groups of Robert B. Woodward in the USA and Robert Robinson in England.
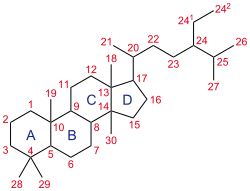
Structure of steroids
Structure
The basic structure of the steroids is the sterane. A structural common feature is the cyclopentanoperhydrophenanthrene ring (exception: vitamin D). Steroids have a rigid molecular shape, usually a relatively high melting point and can be easily crystallized. Due to the asymmetric C atoms at the ring junctions, numerous structural isomers are possible, which are folded differently. Not all possible folds occur in nature. By general convention, the position of the methyl group at carbon atom 10 serves as a reference point for the systematic naming of the isomers: substituents trans to the methyl group are designated by the subscript α (alpha), cis substituents by β (beta). In bile acids, for example, rings A and B are cis-linked (90° angulation), they belong to the 5β-androstanes. Steroid hormones, on the other hand, are trans-linked at this point (5α-androstanes). Secondary groups are abbreviated (e.g. "-ol" = alcohol group). The position of double bonds is indicated with a Δ (delta). The systematic name of cholesterol is, for example, cholest-Δ5-en-3β-ol.
Biosynthesis of steroid hormones
The biosynthesis of steroids is initially similar to the biosynthesis of terpenes. An important intermediate step leads to squalene, a triterpene. Lanosterol is formed by several cyclic linkages. This steroid with a sterane backbone yields cholesterol by cleavage of three methyl groups, hydrogenation and isomerization. Through three different pathways, cholesterol gives rise to aldosterone, testosterone, and cortisol. This occurs in the adrenal cortex and in the male and female gonads (testes and ovary). In the ovary, testosterone (male sex hormone) is also produced first, which is then converted to estradiol by an aromatase (enzyme that dehydrates ring A of the steroid skeleton to a benzene ring). The enzymes that catalyze the steps from cholesterol to steroid hormones can be disrupted by genetic defects. Relatively common is the 21-hydroxylase deficiency. This leads to an overproduction of sex hormones because the pathway to cortisol and aldosterone is disturbed. The disease is called adrenogenital syndrome.
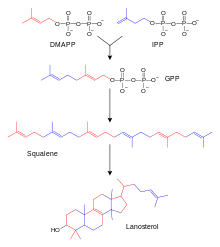
Schematic representation of the lanosterol synthesis.
Questions and Answers
Q: What is a steroid?
A: A steroid is an organic compound, either natural or man-made, which has four cycloalkane rings in its structure.
Q: Where are natural steroids found?
A: Plants, animals, and fungi make hundreds of different natural steroids.
Q: What are steroid hormones?
A: Steroid hormones are steroids that act as hormones in the body.
Q: How are natural steroid hormones usually made?
A: Natural steroid hormones are usually made from cholesterol in the adrenal glands and gonads.
Q: What is the importance of cholesterol in animal cells?
A: Cholesterol is an important steroid in animal cells, as it is necessary for cell membranes.
Q: Can you give examples of steroid hormones?
A: Examples of steroid hormones include estrogen, testosterone, progesterone, Vitamin D3 or cholecalciferol.
Q: What is Prednisone and what is it used for?
A: Prednisone is an important man-made steroid medication used to treat inflammation.
Search within the encyclopedia