Sterilization (microbiology)
![]()
The title of this article is ambiguous. For other meanings, see Sterilization (disambiguation).
![]()
The articles disinfection, hand disinfection, hand sanitizer, sterilization, antiseptic, antisepsis, preservation and preservative overlap thematically. So information you are looking for here may also be in the other articles.
You are welcome to participate in the relevant redundancy discussion or to help directly to merge the
articles or to better distinguish them from one another (→ Instructions).
Sterilization, sterilization and sterilization are processes by which materials and objects are freed from living microorganisms including their resting stages (e.g. spores). The condition of the materials and objects thus achieved is referred to as "sterile".
The term 'germ-free', which is also used instead of 'sterile', is not expressed precisely enough, because sterilisation is not just a matter of removing or killing certain developmental stages of micro-organisms, namely germs, but of removing or killing all micro-organisms at every stage of development. The term "germ-free" is related to the misnomer "germ" for microorganisms at any stage of development.
During the sterilization of materials (e.g. food, pharmaceuticals, solutions), medical instruments, implants, objects, packaging, devices (e.g. endoscopes) and vessels (e.g. for the culture of microorganisms), all contained or adhering microorganisms including their permanent forms (e.g. spores) are (ideally) killed and viruses, prions (infectious proteins), plasmids and other DNA fragments are destroyed.
In practice, complete sterilization does not succeed with 100% certainty. Therefore, a reduction of the number of reproducible microorganisms by a factor determined according to the area of application (in powers of ten) or a certain probability of complete sterilization is required. For example, it is required that the residual content of reproducible microorganisms in a unit of the sterilization material is at most 10-6, i.e.: only one reproducible microorganism may be contained in one million equally treated units of the sterilization material.
Sterilization is carried out by physical (thermal, irradiation) or chemical processes.
In the technical distinction from disinfection, sterilization usually requires a probability of complete sterilization that is higher by a power of ten.

Large sterilization plant (1956)

Apparatus for sterilizing surgical instruments in the administration building of the Schweiz. Hospital and Relief Institute, 1914-1918
Thermal sterilization
For sterilization by heating, the death kinetics of microorganisms is important. The death in a microorganism population is similar to the decay of radioactive elements in that the number of survivors in non-proliferating microorganisms decreases exponentially with time (like the number of atoms of a radioactive element that have not yet decayed). In each unit of time, the proportion of dead individuals in a population is the same.
Decimal reduction time
The time in which nine tenths of the population die, i.e. the population is reduced to one tenth, is referred to as the decimal reduction time D (D value). This time is strongly dependent on the species or strain of the microorganism, the temperature and other conditions, especially the water activity, the pH value and the ionic strength. The decimal reduction time is referred to as 
with initial bacterial count 


Typically, sterilization requires that the number of living individuals be reduced by six powers of ten (i.e., to one millionth, 

The decimal reduction time 

| Bacteria species | D121 °C / minutes |
| Bacillus subtilis | 0,4–0,8 |
| Bacillus cereus | 0,03–2,3 |
| Bacillus stearothermophilus | 2,0–5,0 |
| Bacillus polymyxa | ~0,005 |
| Clostridium botulinum | 0,1–0,2 |
| Clostridium sporogenes | 0,1–1,5 |
| Clostridium thermosaccharolyticum | 69–70 |
| Desulphotomaculum nigrificans | 2,0–3,0 |
The decimal reduction times are considerably higher for bacteria endospores in the dry state. Therefore, higher temperatures and longer exposure times are required for sterilization in the dry state (see "Heating in the dry state" below).
For an individual, this die-off kinetics means: the probability that it will be killed when heated during time period D is always 90 %, the probability of survival is 10 %. The probability that it will be killed in the next time period D is again 90 %, so the probability that it will be killed in time 2 D is always 99 %.
This shows: A complete certainty that after a certain heating time all microorganisms in a sterilization material are killed cannot be achieved. If after heating only 1 individual capable of reproduction is still present, then after further heating for a duration of D it is not certain that this individual is killed, but the probability is only 90%, in 10% of the cases the individual survives this heating. Further heating for a period of 2 D only increases the probability of killing to 99%, in 1% of cases the individual survives this heating also, and so on. Sterilization of a sterilized item can therefore only be achieved with a certain probability.
For example, canned foods can never be sterilized with complete certainty by heating. It is clear from the above that the initial content of heat-resistant bacterial endospores is of great influence. The time required to heat to a given temperature depends on the desired probability of complete sterilization and on the initial content of bacterial endospores. If there are initially about 104 endospores per can, then after a heating time of 4 D, an average of one endospore per can will survive, a totally inadequate result. At 5 D more than 90 % of the cans are sterile, at 6 D 99 %. This may be sufficient for canned goods under certain circumstances, but it is often insufficient for other sterilized goods, for example infusion solutions for medical use. With an initial content of 105 endospores, a heating time of 7 D is required to achieve the same level of safety.
12D concept
The so-called 12D concept is often used for the heat sterilization of canned food. Here it is assumed that each portion (can) contains no more than about 106 bacterial endospores (the most heat-resistant life forms) before sterilization and that the portions should be sterile with a probability of 1 × 106 (this means that out of one million portions only one portion should still contain living microorganisms, i.e. the microorganism content per portion should be 10-6). Then the content of endospores is to be reduced by 12 powers of ten by heating. The heating time tT required at the temperature T is therefore
For the selection of the value for DT: In practice, mixed populations are almost always present in the sterilization material, whose components have different DT values. Since, furthermore, in practice the microorganism contents must be reduced over a large number of powers of ten, the heating time must be calculated with the highest DT value of those microorganisms which are contained in a non-negligible concentration, even if the content of significantly more heat-sensitive microorganisms, i.e. with significantly lower DT, is considerably higher. For example, suppose the content of microorganisms with a DT = 1.0 is 106, and that of microorganisms with a DT = 2.0 is 101. Then, for the reduction to a content of 10-6 in each case, the associated heating times tT for each group are 12 × 1.0 = 12 min and 7 × 2.0 = 14 min. Although the content of more heat-sensitive microorganisms (DT = 1.0) is hundreds of thousands of times higher than that of the doubly heat-resistant ones (DT = 2.0), the F value must still be calculated with the D value of the more heat-resistant microorganisms, otherwise the desired probability of sterility is not achieved.
z-value
The die-off rate of microorganisms increases with temperature, so the D-value decreases. The dependence of the D-value on the temperature is characterised by the z-value. It indicates the amount by which the temperature must be increased in order to reduce the D-value to one tenth, i.e. to increase the killing effect tenfold. The following formula applies for this:
Like the D-value, the z-value is also characteristic for different microorganisms.
F-value
→ Main article: F-value
Sterilization by heating involves a heating phase and a cooling phase, during which lethal temperatures are also reached, but which are not as effective as the higher temperature of the holding phase. However, these phases also contribute to the sterilization effect. In order to have a simple measure for the total effect, the F-value was introduced. It is a measure of the sum of all killing effects during the entire heating process, including heating, holding and cooling phases, expressed as a time equivalent for a reference temperature. The killing effects are lower in the heating and cooling phases than in the holding phase because of the lower temperatures, and depend on the z-value that is characteristic for the respective microorganisms.
In practice, 121.1 °C (corresponding to 250 °F) is selected as the reference temperature and 10 as the z value. The resulting value is referred to as the F0 value. It is given in minutes. An F0 value of 8 thus means that the entire heating process has the same killing effect on microorganisms with a z value of 10 as heating for 8 min at 121.1 °C.
C-value
Important in this context is the C-value (cooking). C(z) = t X 10^((T-RT)/z), where z is the z-value, t is the time in minutes, T is the treatment temperature, RT is the reference temperature and C is the cooking value in minutes.
Heating in the moist state: Steam sterilization
Steam sterilization (heating in an autoclave) is the standard procedure in most laboratories and hospitals (CSSD) and is also used for preserving food in cans and glass packaging. In this process, the food to be sterilized or filled is heated to 121 °C at two bar pressure in steam for 20 minutes or to 134 °C at 3 bar for 5 minutes. Prions are destroyed by heating to 134 °C at 3 bar for 18 minutes.
The air inside the autoclave is completely replaced by water vapor. The actual duration of a sterilization process depends on various technical designs of the autoclaves, such as size, heating capacity, vacuum pumps and other technical factors. The autoclaves fall under the Pressure Equipment Directive and Medical Devices Act or Medical Devices Operator Ordinance and therefore require constant technical monitoring and safety checks.
See also: Sterilizer
| Heat resistance | |||
| Resistance level | Organism/disease agent | Temperature (°C) | Time (min) |
| I | Pathogenic streptococci, listeria, polioviruses | 61,5 | 30 |
| II | most vegetative bacteria, yeasts, | 80 | 30 |
| III | Hepatitis B viruses, most fungal spores. | 100 | 5–30 |
| IV | Bacillus anthracis spores | 105 | 5 |
| V | Bacillus stearothermophilus spores | 121 | 15 |
| VI | Prions | 132 | 60 |
Heating in dry state: hot air sterilization
- The annealing of metallic objects by red heat, about 500 °C, is common in microbiological laboratory work.
- Flaming is a short pulling of the object through a flame.
- Hot-air sterilization for glass, metals, porcelain ("baking"), for
- 180 °C at least 30 min,
- 170 °C at least 60 min,
- 160 °C at least 120 min.
Equipment used for this purpose:
- Hot air sterilization oven for discontinuous sterilization
- Hot air sterilization tunnel for continuous sterilization
- conventional heating, 240 to 320 °C
- injected hot air, 300 to 400 °C
- Laminar flow hot air
Fractionated sterilization
Fractionated sterilization is also named Tyndallization after the Irish physicist John Tyndall. It can only be used for sterilization material in which heat-resistant stages of the microorganisms present (e.g. bacterial endospores) can germinate. The sterilization material is heated to about 100 °C on several consecutive days and stored at room temperature in between. During intermediate storage, the spores not killed by heating should germinate and the resulting non-heat-resistant microorganism stages should be killed during heating the next day. The procedure must be repeated to ensure that all spores germinate and, if necessary, any newly formed spores between two heatings are also re-germinated and killed.

Steam sterilizer

Hot air sterilizer
.JPG)
Autoclave
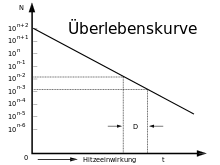
Survival curve
Chemical sterilization
The term chemical sterilization (also known as gas sterilization) refers to sterilization with certain chemical substances such as formaldehyde, ethylene oxide or peracetic acid. In this case, it must be ensured that the sterilization material to be processed is clean and dry and, in addition, has been packed in specially gas-permeable foils. Chemical sterilization is generally used for thermolabile materials such as endoscope optics. For thermostable materials, steam sterilization is always preferable to chemical sterilization.
Wet antiseptic
The microorganisms are killed by chemicals which are applied in liquid form to the objects to be sterilized. For example, in beverage technology, beverages are sterilized by cold sterilization or containers are sterilized with hydrogen peroxide, dissolved ozone or peracetic acid. A critical parameter in all wet antiseptic processes is the temperature of the sterilizing solution. As a rule, the exposure time required for sterilization can be drastically reduced by increasing the temperature. To remove the chemicals from the sterilized object, it is typically washed with sterile water afterwards.
Dry antiseptic
The term "dry antiseptic" refers to a group of sterilization processes that are not sharply defined. The destruction is carried out with gases that act on the dry objects to be sterilized. Gas sterilization is carried out, for example, with formaldehyde, ethylene oxide, ozone or hydrogen peroxide.
It is often used in the cold antiseptic filling of foodstuffs, especially beverages: The objects to be sterilized, usually plastic bottles made of PET or HDPE, are first washed with killing chemicals, such as peracetic acid products in particular, before they are filled (wet antiseptic) and then further killing of microorganisms takes place with gases, preferably by means of hydrogen peroxide added in gaseous form. In contrast to wet antiseptics, the surfaces to be sterilized are dry after sterilization, which is a considerable advantage. The equipment required and the operating costs are generally lower with dry antiseptics than with wet antiseptics. However, the processes are technically more difficult to master and require significantly more know-how.
See, for example, hydrogen peroxide sterilization, a dry antiseptic sterilization process that produces a reduction in survivors of well over 106 in a fraction of a second, even on extremely resistant endospores, but in a vacuum.

EO - sterilized with ethylene oxide, disposable material
EO - sterilized with ethylene oxide, disposable material

Chemiclav
Search within the encyclopedia


