Solid
![]()
This article describes the state of matter. See also: Rigid body in mechanics or volume solid in coating technology.
In the natural sciences, solid refers to matter in a solid state of aggregation. In a narrower sense, this is also understood to mean a substance that has a solid state of aggregation at a temperature of 20 °C, whereby the term solid in this case is substance-specific, but not temperature-specific. In technical language, solids have a certain minimum expansion, but this is not sharply defined. Accordingly, they are macroscopic bodies - in contrast to microscopic bodies. For example, a macromolecule by itself is not usually considered a solid. Matter in the transition area is called a cluster.
Real solids are deformable (elastic or plastic) by forces, unlike idealized rigid bodies. All solids are composed of building blocks. Such elementary parts can be single atoms or molecules, but also groups of them. If all building blocks are of the same kind, one speaks of monostructures, otherwise of heterostructures. The properties of solids differ considerably from the properties of free particles or solutions due to the mutual interaction of the building blocks of matter. A special characteristic of solids is the constancy of the order (amorphous or crystalline) of their building blocks. A different structure of the same building blocks - the modification - significantly affects the properties of the solid.
Solid state physics deals with the physics of matter in the solid state, as a special case of condensed matter, which includes liquids. Materials science is mainly concerned with the physical and chemical properties of solids. Solid state chemistry is particularly interested in the synthesis of new materials in addition to the chemical composition of existing ones. The disciplines are not sharply delimited from each other as well as from mineralogy, metallurgy and crystallography.
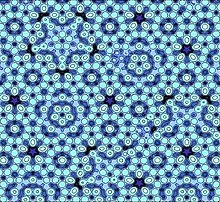
Quasi-periodic crystal as studied by Nobel Laureate in Chemistry (2011) Dan Shechtman.
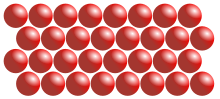
Particle model of a solid
Types
A distinction is made between amorphous (on the smallest scale "shapeless") and crystalline (consisting of crystals) solids. Solid state physics is mainly concerned with the properties of monocrystalline and polycrystalline solids.
Single crystals
In single crystals, the whole body consists of a single crystal. There is a regular, or more precisely a periodic in all dimensions, arrangement of its building blocks. The nature of the underlying structure is responsible for many properties of a solid. For example, carbon has two different crystal structures - graphite and diamond - which have completely different electrical conductivities (graphite conducts electricity, diamond is an insulator). Some minerals occur as natural single crystals with a characteristic external shape.
Quasicrystals
Discovered by Dan Shechtman and awarded the Nobel Prize in Chemistry in 2011, quasicrystals belong to a new type of solid. Quasicrystals are aperiodic, but have a close order with a five-, eight-, ten- or twelve-fold symmetry. Examples of systems with a quasicrystalline structure include aluminum-metal alloys and Cd5,7Yb, Cd5,7Ca in an icosahedral structure, and Ta1,6Te in a dodecahedral structure. Because this phase is only stable in very narrow mixing ranges of the elements, quasicrystals can usually also be counted among the intermetallic compounds.
Amorphous solids
The physics of amorphous solids is complex because it includes all solids that do not have a regular structure. Most glasses or some solidified liquids are only some representatives of this type. With the loss of a macroscopic order, many typical properties of a crystal are also lost. For example, most amorphous solids are good insulators of electricity and heat and are often brittle. Nevertheless, this type of solid represents an interesting field of research, since a lack of crystal structure also means a lack of anisotropy effects. Amorphous phases are usually in a "frozen" metastable state and change their structure and properties with higher temperatures.
Polycrystalline solids
Crystalline and amorphous are not the only possible manifestations of solids. In between there is a range that is, in a sense, a hybrid form: The polycrystalline solids. These consist of a collection of small single crystals that are connected in a disordered manner to form a large whole. In metals, but also in geology, the individual crystallites are often referred to as grains, which are separated from each other by disordered grain boundaries. Together they form a solid structure, which in marble, for example, can be recognized by the sparkling of different grains. The texture describes the orientation of the totality of grains in the solid and is a measure of the anisotropy of many chemical and physical properties.
In polymers, the proportions of crystalline and amorphous phases are described by the degree of crystallization.
Ties
The cohesion of a solid is based on an attractive interaction between the atoms or molecules over long distances and a repulsive interaction over short distances. The energetically most favourable distance is called the equilibrium distance. If the thermal energy of the atoms is too low to escape this potential trap, rigid arrangements are formed - the atoms are bound to each other. The equilibrium distances thus assumed are characteristic of the substance in question and are typically in the range of about 0.1 nm to 0.3 nm. In this order of magnitude, the unit Ångström is very common (0.1 nm = 1 Å).
There are essentially four types of bonds that have a significant influence on the structure and properties of a solid:
Ionic Bond
The ionic bond always occurs - at least proportionally - when the solid is composed of different elements which have different electronegativities. The more electropositive element gives up an electron to the more electronegative one, so one becomes an anion and the other a cation. Different charges cause an electrostatic attraction, while equal charge carriers repel each other. In solids, therefore, anions and cations alternate or form a shell around one another. Salts are typical representatives of this type of bond.
Covalent bond
The covalent bond, also called an atomic bond, is based on lowering the potential energy of the electron states. The orbitals of the two bonding partners overlap and deform to allow an arrangement with as many low energy states as possible. The principle is the same as in the formation of molecules (e.g. O2). Elements of the fourth main group (carbon, silicon, germanium) are bound in this way. The state of the electrons is then called sp3 hybridization. Molecules consist of chains of covalent bonds that influence each other and can be divided into different conformations or generally stereoisomeries.
Metal binding
The metal bond is an extreme case of the covalent bond. This bond is also caused by a lowering of the potential energy of the electron states. Only here the overlap of the orbitals of the atoms is so large that they also interact with those of their next but one (or even more) neighbours. For a cluster, a long-range order that can be described by a lattice (crystal structure) is often the most energetically stable. Some electrons are delocalized, and cannot be assigned to a nucleus. Delocalized electrons can pass energy very quickly through plasmons. Figuratively speaking, the ion hulls of atoms are embedded in an electron lake. As the name suggests, metals form this bond.
Van der Waals link
Van der Waals interactions always occur in principle, but they are so weak that they only become so noticeable in the absence of other types of bonding that one can speak of regular Van der Waals bonds. The attractive force here is a component of the total electrostatic interactions, which decreases with the reciprocal distance to the 7th power and is caused by locally induced dipole moments in the electron density. Noble gas and molecular crystals are held together only by these. Van der Waals interactions are among the dispersion interactions caused in the band or orbital model from 2nd and higher order perturbation theory contributions of interelectronic repulsion.
These types of bonds are by no means isolated cases that occur only either-or. The transition from ionic to covalent to metallic bonding is fluid. In addition, different bonds can occur side by side in solids. Graphite, for example, consists of layers of covalently bonded carbon atoms, while the layers as a whole hold together via van der Waals bonds. Because the latter bond is so weak, graphite is used as pencil lead - when rubbed over paper, the bonds already break. Crystal structures with different bond types are called heterodesmic, those with only one bond type are called homodesmic. A simple mathematical model for the potential energy of two neutral bonding partners (atoms or molecules) is the Lennard-Jones potential.
Questions and Answers
Q: What is solid?
A: Solid is one of the four common states of matter.
Q: How are the molecules in solids arranged?
A: The molecules in solids are closely bound together.
Q: What kind of movement do molecules in solids exhibit?
A: Molecules in solids can only vibrate.
Q: What is the defining characteristic of the shape of solids?
A: Solids have a definite shape that only changes when a force is applied.
Q: How do liquids and gases move compared to solids?
A: Liquids and gases move randomly, a process called flow.
Q: What is the process called when a solid becomes a liquid?
A: When a solid becomes a liquid, this is called melting.
Q: What is the process called when a solid turns directly into gas?
A: Some solids, like dry ice, can turn into gas without turning liquid first. This is called sublimation.
Search within the encyclopedia