Protonation
In chemistry, protonation is the addition of protons (hydrogen nuclei/cations) to a chemical compound as part of an acid-base reaction. Depending on the number of protons transferred, one or more positive charges are added to the target molecule. The compound that has picked up the protons is called the protonated compound. The opposite process, that of the elimination of protons from a compound, is called deprotonation.
Protonation of compound B by the acid HA, which is deprotonated in the process.
The prerequisite for the process of protonation is the presence of an acid and a base according to the definition of Brønsted and Lowry. The acid strength - represented by the pKS value - and the base strength (pKB) determine whether the equilibrium is on the side of the protonated or unprotonated compound.
The protonation of a compound can be influenced by steric factors.
A positive charge is transferred with the proton, as in the following example showing the protonation of ammonia (NH3) by hydrogen chloride (HCl):
Hydrogen chloride donates a proton to the ammonia molecule. This forms a negatively charged chloride anion and a positively charged ammonium cation.
Protonation is a widely observed and used reaction step. It is often used to activate a chemical compound for subsequent reactions. However, it is also used to ionize compounds, for example in the context of mass spectrometric analysis.
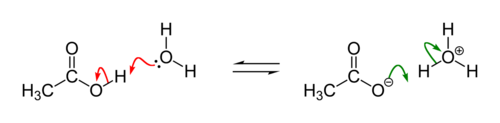
Example: acid-base reaction of acetic acid and water. Red arrows: Deprotonation of acetic acid; green arrows: Protonation of acetate to form acetic acid.
See also
- Protolysis
Search within the encyclopedia

