Avogadro constant
The Avogadro constant 

which is a good 602 quadrillion particles per mole. Generally applies

where 

The unitless number 6.02214076e23 is called the Avogadro number. It was fixed at this value as part of the revision of the International System of Units in 2019 and has since defined the unit of measurement "mole". The number was chosen so that 1 mole of particles, each with a mass of X atomic mass units (u), would have a total mass as close as possible to X grams (g).
Until 2019, the mole was defined by the microscopic and macroscopic mass scales: the amount of substance of a total mass X g of particles of particle mass X u was defined as 1 mole. The Avogadro constant was defined as the number of particles in 1 mol and thus a natural constant to be determined experimentally.
Applications
The Avogadro constant NA is used to convert between quantities that refer to numbers of particles and those that refer to quantities of substances.


Relationships with other constants:

Boltzmann constant

elementary charge

atomic mass
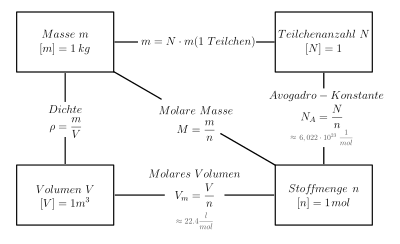
The relationship between mass, amount of substance, volume and number of particles
Search within the encyclopedia






