Photosynthesis
![]()
This article is about photosynthesis as a biochemical process. For the game of the same name, see Photosynthesis (game).
Photosynthesis (ancient Greek φῶς phō̂s, German 'Licht' and σύνθεσις sýnthesis, German 'Zusammensetzung', also spelled photosynthesis) is a physiological process for the production of energy-rich biomolecules from energy-poor substances with the aid of light energy. It is carried out by plants, algae and some bacteria. In this biochemical process, light energy is converted into chemical energy with the help of light-absorbing pigments such as chlorophyll. This energy is then used to build energy-rich organic compounds (primarily carbohydrates) from energy-poor inorganic substances (carbon dioxide (CO2) and water (H2O)). Since the energy-rich organic substances become components of the living organism, their synthesis is called assimilation.
A distinction is made between oxygenic and anoxygenic photosynthesis. In oxygenic photosynthesis, molecular oxygen (O2) is produced. In anoxygenic photosynthesis, which is only carried out by some bacteria, other inorganic substances are produced instead of oxygen, for example elemental sulphur (S).
Photosynthesis is the only biochemical process in which light energy, mostly solar energy, is converted into chemically bound energy. Almost all heterotrophic organisms (organisms that are not capable of photosynthesis) depend indirectly on photosynthesis, since they ultimately owe their food and also the oxygen necessary for energy production through aerobic respiration to it. Oxygen is also the source of the protective ozone layer.
The UVB-dependent formation of cholecalciferol (vitamin D) is also called photosynthesis.
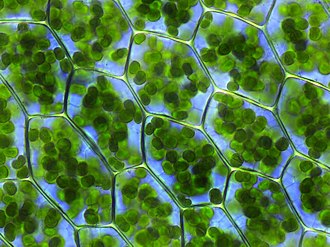
In land plants, photosynthesis takes place in the chloroplasts, here in the leaf blade of the deciduous moss Plagiomnium affine.
Overview
Photosynthesis can be divided into three steps:
- First, the electromagnetic energy is absorbed in the form of light of suitable wavelength using dyes (chlorophylls, phycobilins, carotenoids).
- Directly after this, in the second step, the electromagnetic energy is converted into chemical energy by transferring electrons that have been brought into an energy-rich state by the light energy (redox reaction) (see phototrophy).
- In the final step, this chemical energy is used to synthesize energy-rich organic compounds, which are used by living organisms both in the building metabolism for growth and in the energy metabolism for the production of energy.
The first two steps are called the light reaction and occur in photosystem I and photosystem II in plants. The last step is a largely light-independent reaction.
The synthesis of energy-rich organic substances is predominantly based on the carbon compound carbon dioxide (CO2). For the utilization of CO2, it must be reduced. The electrons of oxidizable substances serve as reducing agents (reductants, electron don(at)ors): Water (H2O), elemental molecular hydrogen (H2), hydrogen sulfide (H2S), divalent iron ions (Fe2+) or simple organic substances (such as acids and alcohols, e.g. acetate or ethanol). In addition, electrons can also be obtained from the oxidation of simple carbohydrates. Which reductant is used depends on the organism, on its enzymes, which are available to it for the use of the reductants.
| inorganic electron don(at)ors of photosynthesis | ||
| Electrondon(at)or | Photosynthesis form | Occurrence |
| Iron II ions (Fe2+) | anoxygenic photosynthesis | Purple Bacteria |
| Nitrite (NO2-) | anoxygenic photosynthesis | Purple Bacteria |
| elemental sulphur (S0) | anoxygenic photosynthesis | Purple Bacteria |
| Hydrogen sulfide (H2S) | anoxygenic photosynthesis | green non-sulfur bacteria, green sulfur bacteria, purple bacteria |
| Thiosulphate (S2O32-) | anoxygenic photosynthesis | Purple Bacteria |
| Water (H2O) | oxygenic photosynthesis | Cyanobacteria, plastids of phototrophic eukaryotes |
| Hydrogen (H2) | anoxygenic photosynthesis | green non-sulfur bacteria |
Photosynthesis balance
The overall reaction scheme of photosynthesis, in the case of CO2 as the starting material, can be formulated in a general and simplified way with the following summation equations, in which <CH2O> stands for the energy-rich organic matter formed.
With a reductant that reduces by giving off hydrogen (H), such as water (H2O), hydrogen sulfide (H2S), and elemental molecular hydrogen (H2), (all symbolized here by the general term <H>):
With a reductant that reduces by donating electrons (e-), such as divalent iron ions (Fe2+) and nitrite (NO2-):
Some bacteria use organic compounds as reductants, such as lactate, the anion of lactic acid:
The overall reaction of photosynthesis with water or hydrogen sulfide as reductant can also be formulated by the following general simplified summation equation:
As a general formulation, H2A stands here for the reductant H2O or H2S.
All algae and green land plants use only water (H2O) as reductant H2A. Cyanobacteria also predominantly use water as reductant. The letter A in this case stands for the oxygen (O) bound in water. It is released as an oxidation product of water during the so-called oxygenic photosynthesis as elemental, molecular oxygen (O2). All oxygen present in the earth's atmosphere and hydrosphere is formed by oxygenic photosynthesis.
The photosynthetic bacteria (Chloroflexaceae, Chlorobiaceae, Chromatiaceae, Heliobacteria, Chloracidobacterium) can use a much wider range of reductants, but predominantly they use hydrogen sulfide (H2S). Many cyanobacteria can also use hydrogen sulfide as a reductant. In this case, since A stands for sulfur bound in hydrogen sulfide, this type of bacterial photosynthesis releases elemental sulfur (S) and not oxygen. This form of photosynthesis is therefore called anoxygenic photosynthesis.
Some cyanobacteria can also use divalent iron ions as reductants.
Even though different reductants are used in oxygenic and anoxygenic photosynthesis, both processes have in common that electrons are gained by their oxidation. Using these electrons, which are brought to a high energy level (low redox potential) with light energy, the energy-rich compounds ATP and NADPH are formed, by means of which energy-rich organic substances can be synthesized from CO2.
The carbon required in the synthesis of energy-rich organic compounds can be obtained from carbon dioxide (CO2) or from simple organic compounds (e.g. acetate). In the first case, we speak of photoautotrophy. The vast majority of phototrophic organisms are photoautotrophic. Photoautotrophic organisms include, for example, all green land plants and algae. In them, a phosphorylated triose is the primary synthesis product and serves as the starting material for the subsequent buildup of building and reserve materials (i.e., various carbohydrates). Photoautotrophs drive (directly and indirectly) nearly all existing ecosystems with their photosynthetic metabolism, as they provide energy-rich building materials and energy sources to other organisms by building organic compounds from inorganic CO2. If simple organic compounds are used as starting materials, this process, which only occurs in bacteria, is called photoheterotrophy.
Research History
Since ancient times (Aristotle), the idea has prevailed that the plant takes its nourishment from the earth. It was not until 1671 that Marcello Malpighi subjected this view to experimental testing, coming to the conclusion that the food juice in the leaves is processed ("cooked out") by the power of sunlight and only in this way can cause growth. Following the discovery of oxygen in the 1770s, Jan Ingenhousz showed in 1779 that it is formed in green leaves when they are exposed to light. In another publication in 1796, he found that the plant takes carbon as food from the "carbonic acid" (carbon dioxide) it ingests and "exhales" the oxygen.
Despite these findings, the humus theory was able to hold on until the middle of the 19th century, because most researchers were convinced that living things can only come from living things. It was not until Justus von Liebig's successes (1840) with mineral fertilizers that it became indisputable that plants could assimilate inorganic substances. In the 1860s, Julius von Sachs described that chloroplasts accumulate starch in the light, which is presumably formed from sugar as the primary product of photosynthesis.
How the assimilation of carbon dioxide proceeds and how this process is related to the action of light remained unclear for a long time. In addition to the assumption that the carbon dioxide is photolytically split directly by the chlorophyll, Frederick Blackman and Gabrielle Matthaei postulated in 1905 that a distinction should be made between a photochemical light reaction and an enzymatic dark reaction. In 1930, Cornelis Bernardus van Niel proposed, by analogy with his results with sulfur bacteria, that photosynthesis was an exchange of hydrogen between a donor and carbon dioxide as acceptor, the donor being water (in the case of sulfur bacteria, analogously H2S). Robert Hill provided impressive evidence for these theses in 1937 by reporting that isolated chloroplasts form oxygen even in the absence of carbon dioxide when iron salts are present as artificial electron acceptors (Hill reaction). In the course of the 1950s, the details of the light and dark reactions were then elucidated by numerous researchers.
Questions and Answers
Q: What is photosynthesis?
A: Photosynthesis is a process used by plants and some microorganisms to turn carbon dioxide into sugars using sunlight. It converts light energy into chemical energy.
Q: What are the products of photosynthesis?
A: The products of photosynthesis are carbohydrates, which are used by cells as energy and to build other molecules.
Q: How does photosynthesis affect life on Earth?
A: Photosynthesis is vital for life on Earth because it was responsible for introducing free oxygen into the atmosphere. Without it, there would be no life on Earth.
Q: Who uses photosynthesis?
A: Green plants, algae, protists and some bacteria use photosynthesis. Some organisms that get their energy from chemical reactions are called chemoautotrophs and do not use photosynthesis.
Q: Is photosynthesis an exothermic or endothermic reaction?
A: Photosythesis is an endothermic reaction, meaning it takes in heat in order to occur.
Q: What kind of energy does photosythesis convert light into?
A: Photosythesis converts light energy into chemical energy.
Search within the encyclopedia



