Penicillin
![]()
Penicillin is a redirect to this article. For the Japanese band of the same name, see Penicillin (band).
The penicillins or penicillins (singular penicillin, from Latin penicillium, 'brush mould') are a group of antibiotically active substances structurally derived from 6-aminopenicillanic acid. In addition to naturally occurring penicillins, which are formed as secondary metabolites of various Penicillium, Aspergillus, Trichophyton and Streptomyces species, they also include biosynthetically and partially synthetically produced penicillins. The first naturally occurring penicillin to be discovered was isolated from the brush mould species Penicillium notatum.
Penicillin G, a natural penicillin still used therapeutically today, is one of the oldest antibiotics in use. In addition to its great medical benefits, it is also credited with pioneering the scientific use of this group of active substances. After the rediscovery by Alexander Fleming in 1928 of the antibiotic efficacy of penicillins, which had already been recognized and published by Theodor Billroth in 1874, the enormous importance of antibiotics to medicine was recognized, significantly influencing and revolutionizing modern understanding of the importance of bacterial pathogens. In the decades following its discovery, penicillin G helped save countless lives. Although today there are numerous strains of bacteria that are resistant to this antibiotic, it can still be used successfully worldwide.
Penicillins belong to the group of β-lactam antibiotics. The molecular formula is R-C9H11N2O4S, where "R" stands for a variable side chain.
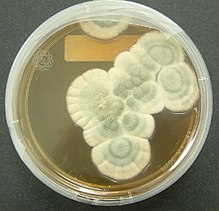
Natural penicillins are produced by molds such as Penicillium chrysogenum
Division
Naturally occurring penicillins
The parent substance of penicillins, 6-aminopenicillanic acid, is formed biologically from L-α-aminoadipic acid and the amino acids L-cysteine and L-valine, with isopenicillin N being formed first. The replacement of the L-α-aminoadipyl residue by other organic acid residues, catalyzed by acyltransferase, gives rise to various penicillins. Of the natural penicillins, only penicillin G (benzylpenicillin), which is obtained fermentatively from Penicillium chrysogenum, is therapeutically significant. It is not degraded as rapidly as the more potent penicillin K. Using a corresponding precursor, phenylacetic acid, it can also be produced specifically by fermentation. In addition to Penicillium species as biological producers, penicillins are also produced by other moulds such as Acremonium chrysogenum (formerly Cephalosporium acremonium) and Aspergillus nidulans, as well as by certain bacteria belonging to the order Actinomycetales, for example Streptomyces clavuligerus produces penicillin N.
| Naturally occurring penicillins | |||||||
|
| |||||||
| Name | Other names | Activity | –R | Sum formula | Molar mass | CAS number | PubChem |
| Penicillin F | Penicillin I, penten(2)yl-penicillin | 1500 I.E./ml | -CH2-CH=CH-CH2-CH3 | C14H20N2O4S | 312.38 g-mol-1 | 118-53-6 | 6438232 |
| Penicillin G | Penicillin II, benzylpenicillin | 1670 I.U./ml (sodium salt) | -CH2-C6H5 | C16H18N2O4S | 334.39 g-mol-1 | 61-33-6 | 5904 |
| Penicillin X | Penicillin III, p-hydroxybenzylpenicillin | 850-900 I.U./ml | -CH2-C6H4-OH | C16H18N2O5S | 350.39 g-mol-1 | 525-91-7 | 120720 |
| Penicillin K | Penicillin IV, heptylpenicillin | 2200 I.U./ml | -CH2-(CH2)5-CH3 | C16H25N2O4S | 341.45 g-mol-1 | 525-97-3 | 44123577 |
| Penicillin DF | Dihydropenicillin F, Penicillin H2F, Amylpenicillin | 1610 I.U./ml | -CH2-(CH2)3-CH3 | C14H22N2O4S | 314.40 g-mol-1 | 4493-18-9 | 107556 |
| Penicillin N | Adicillin | -(CH2)3-CH(NH2)-COOH | C14H21N3O6S | 359.40 g-mol-1 | 71724 | ||
| Penicillin M | Isopenicillin N | -(CH2)3-CH(NH2)-COOH | C14H21N3O6S | 359.40 g-mol-1 | 440723 | ||
Bio- and partially synthetic penicillins
The orally active penicillin V (phenoxymethylpenicillin) is also produced by fermentation, but not spontaneously, but by the addition of a synthetic precursor, phenoxyacetic acid ("biosynthesis").
Partial synthetic further developments of the natural penicillins are produced by the reaction of 6-aminopenicillic acid with carboxylic acid halides. They are characterized by certain advantages over penicillin G, such as acid and penicillin stability or an extended spectrum of activity.
Acid-stable (orally active) penicillins without or with low penicillin stability
- Phenoxymethylpenicillin (Penicillin V), Propicillin, Pheneticillin
Due to a reduced nucleophilicity of the carbonyl oxygen, the attack of hydronium ions is impeded, which inhibits the conversion to the ineffective penillic acid. Acid-stable penicillins are therefore not destroyed by gastric acid and can be administered orally. They have the same spectrum of activity as penicillin G.
Penicillinasestable penicillins
- Cloxacillin, Dicloxacillin, Flucloxacillin, Methicillin, Oxacillin
By shielding the beta-lactam ring with isoxazolyl structures against the penicillinase formed by some pathogens, such penicillins are also effective against penicillinase-forming staphylococci ("staphylococcal penicillin"). Compared to penicillin G, their potency is significantly lower and isoxazolylpenicillins are completely ineffective against Gram-negative pathogens.
Broad spectrum penicillins
- Aminopenicillins: Amoxicillin, Ampicillin, Bacampicillin, Pivampicillin, Sultamicillin.
- Acylaminopenicillins: Azlocillin, mezlocillin, piperacillin...
- Carboxypenicillins: Carbenicillin, Ticarcillin...
- Ureidopenicillins: Azlocillin, Piperacillin, Mezlocillin...
Through the introduction of polar substituents, such hydrophilic penicillins are able to pass through the cell walls of Gram-negative pathogens, thereby expanding their spectrum of activity. The broad-spectrum penicillins are partly acid- and penicillinase-sensitive, and their efficacy against some Gram-positive bacteria is also partly reduced.
No acylation product of 6-aminopenicillanic acid is pivmecillinam, but it has the bicyclic lactam backbone of the penicillins.
Pharmacology
Mechanism of action
Penicillins have a bacteriolytic effect during the cell division of bacteria, namely during the rebuilding of the cell wall, by interfering with the synthesis of the cell wall, preventing the internal cross-linking there and thus weakening the cell wall so that it bursts under stress. In particular, gram-positive bacteria that divide die under the influence of penicillin. The basic structure of penicillins consists of 6-aminopenicillanic acid, a bicyclic dipeptide of cysteine (L-configured) and valine (D-configured, after configuration reversal in the biosynthetic pathway). This so-called beta-lactam ring is bound (in the then opened state) by the bacterial enzyme D-alanine transpeptidase, which is responsible for the cross-linking of peptidoglycans in the bacterial cell walls of Gram-positive bacteria. The enzyme is especially required in dividing bacteria, since in these the rigid cell wall must be opened and at least partially re-synthesized. Since the binding to D-alanine transpeptidase is irreversible, the cell wall can no longer be synthesized and the Gram-positive bacterium loses its most important protective cover. In addition, the constant build-up and degradation of the defective cell wall leads to toxic degradation products.
The effect of penicillins therefore only affects reproducing bacteria, but not non-dividing ones: The antibiotic no longer affects these, because no cell wall synthesis has to take place - it is already complete and therefore no longer forms a point of attack for penicillin. However, bacteria that do not reproduce do not pose a threat to the host organism and are relatively quickly rendered harmless by the patient's own immune defence system. If, on the other hand, they re-enter a multiplication cycle, the cell wall is again partially degraded and must be re-synthesized; such bacteria are therefore attackable again by penicillins. For this reason, penicillins must continue to be administered for a certain follow-up period after the symptoms have subsided.
Penicillins are therefore only effective if the bacteria are otherwise unhindered in their growth; thus, penicillins should not be administered together with drugs that prevent the bacteria from multiplying, since otherwise one would therapeutically block even the starting point of the penicillin's mode of action.
Penicillins not only act on bacteria, including cyanobacteria, but they also block the division of cyanelles, the photosynthetically active organelles of the Glaucocystaceae (an algae family) and the chloroplasts of bladder cap mosses. However, they have no effect on the division of plastids of more highly developed vascular plants, such as tomatoes. This is an indication that in higher plants, due to evolutionary changes in plastid division, β-lactam antibiotics generally no longer have any effect on chloroplasts.
Penicillin G and V are not effective against Gram-negative bacteria (with the exception of Gram-negative cocci such as Neisseria), which have an additional outer membrane above their cell membrane. This makes it impossible for penicillin to attack, as it has to interfere with the formation of the underlying peptidoglycan layer. Therefore, the use of penicillin G and V is only useful against gram-positive bacteria. Structural variants such as aminopenicillins are used against Gram-negative bacteria.
Resistances
Numerous - but not all - clinically occurring bacteria are already resistant to penicillin G today, which has led to a number of further developed β-lactam antibiotics. One exception, for example, is the bacterium Treponema pallidum (causative agent of syphilis/Lues), against which there has been no development of resistance to penicillins to date.
The problem of cross-resistance remains critical, which means that germs that have once developed resistance to penicillins also become insensitive to other β-lactam antibiotics (e.g. cephalosporins).
Resistant mutants would not actually cause any harm, as they occur only in small numbers. However, if the penicillin acts on the other, non-resistant bacteria and eliminates them, a resistant bacterium can reproduce much more easily and thus becomes a danger, as it passes on its resistance to the subsequent generations. Through the exchange of resistance genes between different bacterial species, antibiotic resistance is also passed on to other species. Methicillin resistance in Staphylococcus aureus is particularly feared.
The process of resistance development is a very illustrative example of Darwinian evolutionary theory (natural selection); due to rapid division and generation succession, resistant bacteria adapted to their environment are selected and form the basis for later generations. The formation of penicillin-resistant strains is considered one of the first experimental evidences of observed microevolution. The biological basis of the group of agents is the competition between the two strains of organisms, fungi and bacteria, which depend on the same resources, with the fungi protecting themselves against the bacteria with antibacterial growth-inhibiting substances.
Side effects
As with all antibiotics, resistance can also develop to penicillins. The frequency of allergies to penicillin therapy is much lower than previously assumed (). Allergic reactions can range from mild reddening of the skin to anaphylactic shock.
Penicillins can kill useful bacteria such as those of the intestinal flora, especially broad-spectrum penicillins, which are also effective against Gram-negative bacteria. In the worst case, harmful microorganisms can thus spread in the intestine and lead to antibiotic-associated colitis.
Another rare (with a probability of about three per thousand) adverse effect is the triggering of epileptic seizures.

Pharmacy shop window 1954
Questions and Answers
Q: What is penicillin?
A: Penicillin is a group of common antibiotics used to treat bacterial infections.
Q: When was penicillin discovered?
A: Penicillin was discovered by Scottish scientist Sir Alexander Fleming in 1928.
Q: How did Fleming discover penicillin?
A: Fleming noticed a mold that was stopping bacteria from growing in a petri dish.
Q: Who developed the penicillin mold into a medicine?
A: Australian scientist Howard Walter Florey developed the penicillin mold into a medicine.
Q: For what diseases can penicillin be used to treat?
A: Penicillin can be used to treat syphilis, tonsillitis, meningitis, and pneumonia as well as other diseases.
Q: When did it first become widely used?
A: Penicillin first became widely used during World War II.
Q: What are some symptoms of an allergic reaction to penicillin?
A: Symptoms of an allergic reaction to penicillin include nausea, diarrhea, or rash. Rarely, patients who are allergic may get a fever, vomit, or have serious skin irritation.
Search within the encyclopedia
