Palladium
![]()
The title of this article is ambiguous. For other meanings, see Palladium (disambiguation).
Palladium is a chemical element with the element symbol Pd and atomic number 46. The rare, silvery-white transition metal, together with platinum, rhodium, ruthenium, iridium and osmium, forms the platinum group of metals, gray to silvery-white metals with related chemical and physical properties. In the periodic table, it is in the 5th period and 10th group or nickel group. It was formerly placed in the 8th subgroup.
The metal was discovered in 1802 by William Hyde Wollaston, who was investigating methods of processing platinum ores. He named it after the then newly discovered asteroid Pallas, which at the time was thought to be a planet. The asteroid in turn was named after the epithet of the Greek goddess Athena.
Alongside platinum and rhodium, palladium is an economically important platinum metal and is used in large quantities for the production of three-way catalytic converters. It is also used in electronics, dentistry, fuel cells and many other areas, such as in the jewellery industry, where it is alloyed with gold to form white gold. Extensive deposits have been found in South Africa in the Bushveld complex, in the Stillwater complex in Montana, as well as in Ontario, Russia and the Philippines, where it occurs in pure form as a companion to gold and platinum metals.
History
Palladium was unwittingly used as a component of platinum alloys by the pre-Columbian Indians of Ecuador and Colombia. A number of platinum jewels were found there containing about 85% platinum, 7% iron and 4.6% of a mixture of the platinum metals palladium, rhodium and iridium, and copper.
William Hyde Wollaston discovered palladium in a South American platinum ore in 1802. He had dissolved the ore in aqua regia and then neutralized the solution with sodium hydroxide. He then precipitated the platinum with ammonium chloride as ammonium hexachloroplatinate and separated it. By adding mercury cyanide to the remaining solution Wollaston obtained palladium cyanide, from which he obtained metallic palladium by heating.
As early as 1866, Thomas Graham noted the amazing storage capacity of finely divided palladium for hydrogen, which can hold about 900 times its own volume of hydrogen gas at room temperature and atmospheric pressure. This led to the assumption that hydrogen was a very volatile metal and that the palladium with the trapped hydrogen was an alloy of this volatile metal.
Francis Clifford Phillips, a US chemist, discovered the stoichiometric oxidation of ethene to acetaldehyde using palladium(II) chloride in 1894 when he was investigating the oxidation of naturally occurring hydrocarbons. Towards the end of the 1950s, Wacker-Chemie converted the stoichiometric reaction discovered by Phillips into a catalytic variant in the Wacker-Hoechst process. In the process, which produced millions of tons of acetaldehyde and its downstream product acetic acid per year, the chemical industry used a palladium catalyst in a large-scale application for the first time. It was also the first large-scale homogeneous catalytic process.
From the end of the 1960s, palladium salts were used for coupling reactions. This led to the development of reactions that are important for organic chemistry, such as the Heck reaction, the Stille coupling, the Suzuki coupling and the Negishi coupling. Three of the researchers involved, Richard F. Heck, Ei-ichi Negishi and Akira Suzuki, were awarded the Nobel Prize in Chemistry in 2010.
Electrochemical adsorption experiments in 1989 by Martin Fleischmann and Stanley Pons with the palladium-deuterium system became known as "cold fusion" and hit the headlines worldwide. The supposed "cold fusion" of deuterium triggered by palladium was considered a scientific sensation for a short time with the hope that this could provide a virtually inexhaustible source of energy.

Thomas Graham, lithograph by Rudolf Hoffmann, 1856.
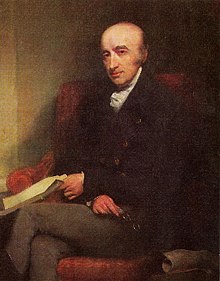
William Hyde Wollaston, circa 1820-1824.
Occurrence
→ Platinum Metals/Tables and Charts
Metallic palladium and palladium-bearing alloys are mainly found in river sediments as geological placers in the Urals, Australia, Ethiopia and in North and South America. However, they have been largely exploited for decades.
Today it is mostly extracted from nickel and copper ores. In 2011, about 41 % (85,000 kg) came from Russian production, followed by South Africa with about 37.5 % (78,000 kg). Canada followed at a great distance with just under 9 % (18,000 kg) and the USA with 6 % (12,500 kg). In the "platinum group of metals" (platinum, palladium, iridium, osmium, rhodium and ruthenium), South Africa has more than 95 % of the world's reserves with 63 million kilograms out of 66 million kilograms worldwide.
With end-of-life vehicle disposal, the proportion of recycled palladium from exhaust catalysts will increase. Di-n-hexyl sulfide can be used to selectively separate palladium from other metals in hydrochloric acid solutions.
.PNG)
Palladium production volumes 2005
Search within the encyclopedia