Nuclear fusion
![]()
This article deals with nuclear physics reactions. For use in electricity generation, see fusion energy.
Nuclear fusion refers to nuclear reactions in which two atomic nuclei each fuse to form a new nucleus. Nuclear fusion reactions are the reason why the sun and all shining stars radiate energy.
Of decisive importance for the occurrence of a fusion is the cross section, the measure for the probability that colliding nuclei react with each other. The cross section is usually only sufficiently large if the two nuclei collide with high energy. This is necessary to overcome the Coulomb barrier, the electrical repulsion between the positively charged nuclei, or to tunnel through its narrow maximum. Beyond the barrier, at a distance of only about 10-15 m, the attraction due to the strong interaction prevails and the nuclei merge.
Fusion reactions can be exothermic (dissipating energy) or endothermic (absorbing energy). Exothermic fusion reactions can maintain the high temperatures necessary for thermal energy to lead to further fusion reactions. Such thermonuclear processes occur in stars and fusion bombs under extreme pressure. Unlike nuclear fission, chain reaction is not possible with fusion reactions.
The fusion reaction shown above as a thermonuclear process is intended to be used in the future to generate electricity in nuclear fusion reactors: Nuclei of deuterium (2H) and tritium (3H) fuse to form a helium nucleus (4He), releasing a neutron (n) as well as energy (3.5 MeV + 14.1 MeV).
The figure below shows the binding energy per nucleon of the nuclides. Energy is released during reactions in the upward direction of the curve or is required in the downward direction. The fusion of hydrogen (H) into helium-4 releases a particularly large amount of energy.
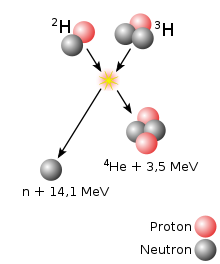
Fusion of deuterium and tritium into a helium nucleus
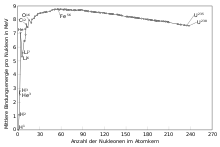
Binding energy per nucleon as a function of mass number
Research into nuclear fusion
Already the first observed nuclear reaction was an (endothermic) fusion reaction. It was discovered - long before nuclear fission - by Ernest Rutherford in 1917 during experiments with alpha particles. Protons of relatively high energy were found, which only occurred when the irradiated gas contained nitrogen. This nuclear reaction is called in today's notation 14N(α,p)17O or, written in detail:
This conversion of nitrogen into oxygen was, like the alpha decay itself, in contradiction to the classical theory, according to which the Coulomb barrier can only be overcome with sufficient energy. It was not until 1928 that George Gamow was able to explain such processes on the basis of the new quantum mechanics with the tunnel effect.
As early as 1920, Arthur Eddington had suggested fusion reactions as a possible source of energy for stars, based on the precise measurements of isotopic masses by Francis William Aston (1919). Since it was known from spectroscopic observations that stars consist largely of hydrogen, its fusion into helium came into consideration here. In 1939 Hans Bethe published several mechanisms how this reaction could take place in stars.
The first targeted fusion reaction in the laboratory was the bombardment of deuterium with deuterium nuclei in 1934 by Mark Oliphant, Rutherford's assistant, and Paul Harteck. The fusion of this hydrogen isotope, which is rare in stars, branches into two product channels:
The technical use of thermonuclear fusion was first pursued with the aim of military weapons development. Therefore, this research took place in secret in the first decades after the Second World War. The USA had been in possession of the nuclear fission-based atomic bomb since 1945, the Soviet Union since 1949. Subsequently, Edward Teller and Stanislaw Ulam in the USA developed a concept for building a hydrogen bomb based on nuclear fusion, which promised a much higher explosive power. On November 1, 1952, the first hydrogen bomb, named Ivy Mike, was detonated at Eniwetok Atoll in the Pacific Ocean. This was proof that large amounts of energy could also be released on Earth through nuclear fusion.
Energy Balance
If the mass of the nuclei or particles created in the fusion is less than the sum of the masses of the initial nuclei, the mass difference Δ 

The experiments to date on controlled thermonuclear fusion do not yet have a positive energy balance. The most successful so far was the British JET (Joint European Torus) facility, which was able to achieve a peak power of 16 MW for less than a second. In the process, 65 percent of the energy put into it could be recovered as fusion energy.
Questions and Answers
Q: What is nuclear fusion?
A: Nuclear fusion is the process of making a single heavy nucleus (part of an atom) from two lighter nuclei. This process is called a nuclear reaction and releases a large amount of energy.
Q: How does this process work?
A: The nucleus made by fusion is heavier than either of the starting nuclei, but not as heavy as the combination of their original mass. This lost mass is changed into lots of energy, which can be seen in Einstein's famous E=mc2 equation.
Q: Where does this process occur?
A: Fusion happens in the middle of stars, such as our Sun, where hydrogen atoms are fused together to make helium and release lots of energy that powers its heat and light.
Q: Are all elements able to be joined through fusion?
A: No, heavier elements are less easily joined than lighter ones and iron (a metal) cannot fuse with other atoms at all. This is what causes stars to die when they join all their atoms together to make heavier atoms until they start making iron which cannot be fused anymore.
Q: Is it easy to start nuclear fusion reactions on Earth?
A: No, it is very difficult because these reactions only happen at high temperature and pressure like in the Sun due to both nuclei having positive charges which repel each other so they must hit each other at very high speeds for them to fuse successfully.
Q: Has anyone been successful in controlling or containing these reactions for electricity generation?
A: Not yet - scientists and engineers have been trying for decades but still have many challenges before fusion power can be used as a clean source of energy.
Q: What has been successful so far with regards to nuclear fusion?
A: The only successful approach so far has been in nuclear weapons where the hydrogen bomb uses an atomic (fission) bomb to start the reaction.
Search within the encyclopedia


