Atomic theory
An atomic model is an idea of the structure and form of atoms. Already in antiquity there was the atom hypothesis, according to which the atoms were regarded as the indivisible and unchangeable basic building blocks of all material substances. At first, the atom hypothesis could only be based on the philosophical preference for a particle model over the hypothesis of the infinitely continuable divisibility of matter. Also, the different properties of material substances were to be attributed to the possibilities of combination of a few kinds of atoms. It was not until the beginning of the 19th century that scientific evidence of the real existence of atoms began to appear in chemistry and physics: The atom was defined as the smallest unit of a chemical element, and the behavior of gases could be explained entirely from the disordered motion of a large number of identical molecules, each consisting of a few atoms, according to the kinetic theory of gases. The idea of a small sphere with a diameter of about 0.1 nm and a mass of about 10-26 kg sufficed as an atomic model. In this form, the atomic hypothesis had become widely accepted by the end of the 19th century, when new observations with electron beams and radioactive substances showed that these atoms themselves consisted of smaller particles. The explanation of their complicated internal structure led in 1925 to quantum mechanics, whose atomic models are primarily formulated as mathematical statements. When asked how to imagine an atom, Werner Heisenberg, one of the creators of quantum mechanics, replied, "Don't even try!"
The following chronologically ordered list gives an overview. Important models have main articles. Currently used models are also presented in the article Atom in context.
- The particle model of Democritus (about 400 B.C.) postulates the existence of various solid, indivisible particles, which in different combinations form the known substances.
- The Dalton model (1803) assumes the smallest, not further divisible particles, which differ in their mass depending on the element and are linked in different substances in certain number ratios (depending on the type of substance). When substances are changed by chemical reactions, the atoms can only rearrange themselves.
- In the dynamid model (1903), atoms consist of small rotating electric dipoles, the dynamids, and the empty space between them.
- According to Thomson's atomic model (1903), the atom consists of an evenly distributed positive charge and negatively charged electrons moving within it. This model is also called the plum pudding model or the raisin cake model. It can explain why atoms are permeable to high-energy particle beams (such as cathode rays, alpha rays), because the positive charge is assumed to be free of matter.
- In the planetary model or Saturn model of Nagaoka Hantarō (1904), the atom consists of a positively charged sphere orbited by the negatively charged electrons. By analogy with the stability of Saturn's rings, the model correctly postulates a very massive nucleus, but incorrectly also postulates energy radiation due to the motion of the electrons.
- In Haas' atomic model of 1910, a quantum condition is introduced for the first time. The hydrogen atom is said to consist of a homogeneously charged positive hull (as in Thomson's model), on the surface of which an electron orbits in a circle. Haas determines the quantity by identifying - without further justification - the energy of this state (potential calculated from the rest position at the center) with the energy of a photon at the short-wave limit of the Balmer series. The same formula results as later in the Bohr atomic model for the size of the first excited state, which is also the ground state of the Balmer series.
- According to the Rutherford model of the atom (1911), the atom consists of a very small positively charged nucleus, which contains almost the entire mass of the atom, and which is surrounded by an atomic shell of electrons in a manner not further described. This explained the observation of the rare strong deflections of alpha particles.
- The Barkl shell model (1912) simplifies the atom in such a way that a positively charged atomic nucleus is surrounded by spherical shells in which the electrons are located. Only the outermost shell is responsible for the chemical properties of the element. No statement is made about the movement of the electrons.
- According to Bohr's atomic model (1913), the atom consists of a positively charged, mass-bearing nucleus and electrons that orbit around it in certain circular paths without radiating energy. With the help of this model, it was possible for the first time to calculate some energy levels of the hydrogen atoms with good accuracy.
Due to the success of the Bohr atomic model, the older models are practically no longer represented. The term "atomic model" subsequently generally refers to a model of the atomic shell. Models for the atomic nucleus were developed by Rutherford from 1919 onwards and are referred to as nuclear models.
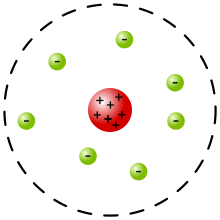
Rutherford's atomic model: The protons are in the atomic nucleus. The electrons are disordered in the atomic shell.

Representation of different atoms in the orbital model. The orbitals are the wave functions of the electrons belonging to the atom.
Models of the atomic nucleus
- The droplet model (1936) describes the atomic nucleus as a droplet of an electrically charged liquid.
- The shell model (1949) describes the atomic nucleus in close analogy to the orbital model of the atomic shell.
Questions and Answers
Q: What is the atomic theory?
A: The atomic theory explains how our understanding of the atom has changed over time.
Q: What were atoms once thought to be?
A: Atoms were once thought to be the smallest pieces of matter.
Q: What are atoms actually made up of?
A: Atoms are made up of protons, neutrons, and electrons.
Q: What are subatomic particles made up of?
A: Subatomic particles are made up of quarks.
Q: Who is the Greek philosopher who first came up with the idea of the atom?
A: The first idea of the atom came from the Greek philosopher Democritus.
Q: Who is the British chemist and physicist who contributed many ideas to the modern theory?
A: John Dalton, a British chemist and physicist, contributed many ideas to the modern theory.
Q: Does the atomic theory apply to plasmas or neutron stars?
A: The theory applies to solids, liquids, and gases, but it does not apply in a way analogous to plasmas or neutron stars.
Search within the encyclopedia