Nitrogen
Nitrogen (Latin nitrogenium) is a chemical element with atomic number 7 and the element symbol N. In the periodic table, it is in the fifth main group or the 15th IUPAC group or nitrogen group and the second period. The symbol N is derived from the Latin nitrogenium (from ancient Greek νίτρον nítron "alkali salt" and γένος génos "origin"). The German name Stickstoff recalls that molecular nitrogen extinguishes flames by displacing oxygen ("suffocates") and that in pure nitrogen living things suffocate because oxygen is absent. Older names are azote, azotum (from ancient Greek ἄζωτος ázōtos "hostile to life"), nitrogen gas, and zoogenium.
Elemental nitrogen occurs only in the form of diatomic molecules (molecular nitrogen, also dinitrogen, molecular formula N2); at 78 %, it is the main constituent of air. Inorganically bound nitrogen rarely occurs in the earth's crust; it is only important in nitrate deposits.
In the course of evolution, a nitrogen cycle of ecosystems has developed: As a component of proteins and many other natural substances, nitrogen is essential for living organisms, which organically bind it in an energy-intensive process (nitrogen fixation) and make it bioavailable. This occurs, for example, enzymatically at an iron-sulfur cluster, which is a cofactor of the nitrogenase enzyme.
History
Naturally occurring chemical compounds of nitrogen, such as nitrates and ammonium salts, were already used in ancient times and by the alchemists. Both types of compounds, in addition to their occurrence as minerals, can also be produced from excrement. For example, the Egyptians produced ammonium chloride (sal ammoniac) from camel dung, and saltpetre was long extracted from the floor of stables. Carl Wilhelm Scheele proved nitrogen to be a constituent of the air in 1771 and this was confirmed by Daniel Rutherford in 1772. Pure ammonia was first shown in 1774 by Joseph Priestley. Until the early 20th century, saltpeter was the only major source of nitrogen compounds. The introduction of the Frank-Caro process (calcium cyanamide production according to Adolph Frank and Nikodem Caro) made atmospheric nitrogen usable for the first time. The Birkeland-Eyde process, after Kristian Birkeland and Sam Eyde, was used to obtain nitric acid. These processes were soon replaced by the Haber-Bosch process, after Fritz Haber and Carl Bosch, for the synthesis of ammonia from atmospheric nitrogen and hydrogen, and by the catalytic Ostwald process, after Wilhelm Ostwald, for the conversion of ammonia to nitric acid.

Carl Wilhelm Scheele
Natural occurrence and cycle of nitrogen
As early as the 19th century, it was recognized that a large part of plant matter contains nitrogen and is an important building element of all living things. It is the essential element of proteins and proteids (proteins) and DNA. Nitrogen is therefore also the building block of all enzymes that control plant, animal and human metabolism. Nitrogen is indispensable for life on earth.
Nitrogen in the air
The Earth's atmosphere consists of 78.09% by volume (75.53% by weight) of molecular nitrogen. Only a small number of microorganisms can use it, incorporate it into their bodily substance or release it to plants. As far as is known, plants cannot directly use the gaseous nitrogen in the air. The conversion into a form that can be used by plants occurs through:
- Nodule bacteria: These bacteria invade the roots of plants called legumes. They feed on the assimilates of the plant. In exchange, they supply the host plant with ammonium. This is reduced from atmospheric nitrogen by a special enzyme, nitrogenase, with a high expenditure of energy. This symbiotic community is a symbiosis. It enables legumes to colonize even poor sites, which is why humans use these plants to enrich the soil with nitrogen, especially in organic farming. Here, legumes are the main source of nitrogen.
- Free-living microorganisms: Non-symbiotic nitrogen fixation is based on the ability of some free-living microorganisms (for example Azotobacter and Cyanobacteria) to use atmospheric nitrogen to build up their own protein. For arable use, the magnitude of atmospheric nitrogen fixation by free-living microorganisms is assumed to be 5-15 kg/ha per year.
- Electrical discharge during thunderstorms: In high rainfall areas, 20-25 kg N per ha per year can be added to the soil by rain. This occurs during electrical discharges when oxygen and nitrogen combine to form nitrogen oxides. Eventually, these oxides react with rainwater to form nitric acid and nitrates can form in the soil.
- Ammonia synthesis: At the beginning of the 20th century, chemists Fritz Haber and Carl Bosch developed a process for producing ammonia from atmospheric nitrogen and hydrogen. The use of atmospheric nitrogen made possible by the Haber-Bosch process has contributed to a substantial increase in agricultural yields. Food security has thus been substantially improved. The plant builds up vegetable protein from the absorbed ammonia, which serves humans and animals as food and for building up their own body protein. In the human and animal organism, the protein is largely broken down again and excreted in the faeces and urine. At the present time, on average, every third nitrogen atom in the biosphere has already been processed once by the fertilizer industry.
- Car exhaust: The combustion of fossil fuels (petrol, diesel) releases nitrogen compounds through car traffic. The combustion process produces nitrogen oxides (NOx, mainly nitrogen dioxide NO2, but also nitrogen monoxide NO and other NOx compounds). In the past, these were released directly into the environment. Nowadays, cars have catalytic converters that reduce these compounds: NOx is reduced to ammonia in the catalytic converter. This is converted to ammonium in the presence of water (ammonia/ammonium equilibrium in acidified solution: NH3 + H3O+ ⇔ NH4+ + H2O). Both the oxidized and the reduced nitrogen compounds are transported via the air and contribute to a considerable extent to the eutrophication of neighbouring ecosystems.
Nitrogen in the soil
In the topsoil (A horizon), more than 95 % of the total nitrogen is usually present as organically bound nitrogen in living root mass, dead plant mass, humus substances and soil organisms. The remainder of less than 5 % is inorganic nitrogen in the form of ammonium or nitrate and in very small quantities in the form of nitrite. This mineral nitrogen content is determined by the Nmin method in the spring before fertilisation. The total nitrogen content of soils is strongly dependent on their carbon content. It is influenced by climate, vegetation, soil type, terrain and measures taken by the farmer, such as tillage.
Nitrogen in plants
Tasks in the plant
Nitrogen is incorporated into the photosynthesis products to produce proteins, among other things, and thus promotes growth. Nitrogen plays an important role as an essential component of deoxyribonucleic acid and chlorophyll. Depending on the species, the proportion of dry matter is 2-6 %, or an average of 1.5 %. The uptake of nitrogen usually takes place in the form of ammonium or nitrate salts.
Symptoms of deficiency
- stunted growth
- pale green color of the leaves. Older ones become chlorotic and fall off prematurely.
- too early flowering (emergency flowering)
- Yellowing
Excess symptoms
- Masty growth
- Leaves dark green
- Flowering delayed
- Plant susceptible to frost and disease
- Leaf tissue appears spongy and soft
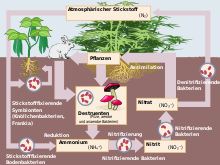
Nitrogen cycle
Questions and Answers
Q: What is nitrogen?
A: Nitrogen is a chemical element that is a nonmetal.
Q: How much nitrogen is in the atmosphere?
A: The atmosphere contains more than 78 percent nitrogen.
Q: What is the chemical symbol for nitrogen?
A: The chemical symbol for nitrogen is N.
Q: What is the atomic number of nitrogen?
A: The atomic number of nitrogen is 7.
Q: How many nucleons does the stable inside of nitrogen typically contain?
A: The stable inside of nitrogen typically contains 14 nucleons, which consist of 7 protons and 7 neutrons.
Q: How many electrons does nitrogen have in its outer shell?
A: Nitrogen has 5 electrons in its outer shell.
Q: Is nitrogen a metal or a nonmetal?
A: Nitrogen is a nonmetal.
Search within the encyclopedia