Nitrate
The salts as well as the esters of nitric acid (HNO3) are called nitrates.
The salts have the general composition MINO3 (MI: monovalent cation). Some of the salts are called by the historical trivial name saltpetre. The planar anion NO3- carries a negative charge.

The mesomeriestabilized nitrate anion.
The total charge is -1.
The esters of nitric acid are also called nitric acid esters and have the general structure R-O-NO2 (R: organic residue).

Nitric acid ester with simplified formula (left) and the structural formula (right).
The radical R is an organyl radical (aryl radical, alkyl radical, arylalkyl radical etc.).
The nitric acid ester group (nitrate group) is marked in blue.
Some nitric acid esters are incorrectly referred to as nitro compounds, e.g. glycerol trinitrate as nitroglycerine. However, unlike nitrates, nitro compounds (R-NO2) have a C-N bond.
In mineralogy, nitrates form a separate class together with carbonates or with carbonates and borates, depending on the mineral system used. In the meanwhile outdated 8th edition of Strunz's mineral systematics according to Dana, which is mainly used in English-speaking countries, carbonates, nitrates and borates are combined in one class, whereas in the modern 9th edition of the mineral systematics according to Strunz, which is used by the International Mineralogical Association (IMA), only the carbonates and nitrates are combined.
Salts of nitric acid
Structure
| Salts of nitric acid | ||
| Name | Formula | Trivial name |
| Potassium nitrate | KNO3 | Potassium nitrate |
| Sodium nitrate | NaNO3 | Sodium nitrate, Chile saltpetre |
| Barium nitrate | Ba(NO3)2 | Barytes nitrate |
| Calcium nitrate | Ca(NO3)2 | Calcium nitrate, masonry nitrate |
| Ammonium nitrate | NH4NO3 | Ammonium nitrate |
| Silver nitrate | AgNO3 | Höllenstein |
| Aluminium nitrate | Al(NO3)3 | |
| ferric nitrate | Fe(NO3)3 | |
| For further examples see category:Nitrate | ||
The nitration is built planar. All bond angles O-N-O are 120°. Similarly, the bond lengths of the N-O bonds are equal and lie between the lengths for single and double bonds, respectively. The real structure of the nitrate ion must therefore exist between three mesomeric boundary structures:

Nitrogen, as an element of the second period, has no octet expansion, so that the mesomeric boundary structures are present with positive and negative charges.
Features
The salts - with the exception of the bismuth oxide nitrate BiONO3 - are readily soluble in water and play an important role as nutrients for plants. Nitrate anions themselves are largely non-toxic. Limits to toxicity to mammals and humans are in the same order of magnitude as those for chlorides and sulfates, namely in the two-digit gram range. However, as with all salts, large amounts lead to osmotic problems (see: Osmoregulation). As food additives, nitrates are used in the milligram range.
During dry heating (melting) nitrates decompose.
Thus, sodium nitrate reacts with oxygen splitting to form sodium nitrite:
Lead nitrate forms lead(II) oxide and nitrogen dioxide is released:
Alkali nitrates are used as oxidizing agents, e.g. in black powder.
Production
Nitrate salts are easily accessible by reacting nitric acid with, for example, hydroxides, carbonates, metals or similar:
Conversion of nitric acid with barium hydroxide to barium nitrate and water.
Conversion of nitric acid with potassium carbonate to potassium nitrate, water and carbon dioxide.
Conversion of nitric acid with zinc to zinc nitrate, water and nitrogen monoxide.
Nitrates are also accessible by complete oxidation of nitrogen compounds such as nitrites, ammonia or hydroxylamine.
Occurrence
Nitrates are ubiquitous in the biosphere and hydrosphere mainly in the form of sodium nitrate. Because of their high solubility, nitrates accumulate in larger quantities only at a few specific locations. Large nitrate deposits worthy of degradation are therefore rare. They are found in deserts with long-lasting hyperarid climates, such as the Atacama Desert in Chile (ca. 2 × 108 t), the Turpan Depression in northwestern China (ca. 2.5 × 108 t), the McMurdo Dry Valleys of Antarctica, and the Mojave in the United States. There are other localities in Egypt, Asia Minor, and Colombia. Nitrates of the common alkali and alkaline earth metals occur in natural form as chile, lime or potassium nitrate.
Formation and degradation of nitrates
→ Main article: Nitrogen cycle
In soil and water, nitrates are formed by bacterial nitrification. During the decomposition of protein-containing substances in particular, primarily ammonium compounds are released. Oxidation by bacteria of the genus Nitrosomonas leads to nitrite, which is further oxidized to nitrate by bacteria of the genus Nitrobacter.
If there is a lack of oxygen, on the other hand, bacterial denitrification of nitrate leads to elemental nitrogen. These conversions are systematically exploited, e.g. in wastewater treatment plants, to eliminate nitrogen compounds.
Use
Nitrate acts as an efficient oxygen donor. This is why potassium nitrate is a component of black powder (explosive nitrate). Nitrates of other cations may also be used, mostly when coloured light effects are desired in pyrotechnics; e.g. barium nitrate (green), and strontium nitrate (red). Barium nitrate is also used in sparklers without producing a noticeable flame coloration.
As a food additive, sodium nitrate (E 251) and potassium nitrate (E 252) are used as preservatives, e.g. for curing meat and sausage products, as they inhibit the growth of anaerobic germs by forming nitrite.
In living organisms, nitrates cause an enzymatic release of the blood-vessel-dilating nitric oxide (NO) and are therefore used in medicine (for example, for the treatment and prevention of angina pectoris attacks and in the treatment of severe acute heart failure), including the drugs nitroglycerin or isosorbide mononitrate for vasodilatation (see also NO donors).
Fertilization
Nitrates are utilized by plants as nutrients and used in agriculture as fertilizers. They can be absorbed and utilized directly by plant organisms as a source of nitrogen.
In agriculture, nitrates are used as fertilizers, also in the form of liquid manure (general farm manure). These farm fertilizers contain nitrogen partly as nitrate (calcium nitrate in blue grain) and partly as ammonium compounds (ammonium nitrate, ammonium phosphate), but often also in the form of organic nitrogen compounds (proteins, amines, urea). Through nitrification, nitrate is formed in the soil from ammonium ions (NH4+) via the intermediate stage nitrite with the help of bacteria. The organically bound nitrogen can be mineralised in the soil (release of ammonium compounds and ultimately also nitrate) or enter the soil humus stock, from which it is only gradually re-mineralised (usually 1 to 3 % mineralisation rate per year).
Particularly as a result of liquid manure management, (in subordinate quantities also through excessive fertilization, e.g. in the cultivation of various types of vegetables or in private home gardens, as well as through leaking wastewater pipes), the nitrate content of groundwater has increased significantly in recent decades. Legal regulations on the type, quantity and timing of fertilization, as well as corresponding further training and remediation measures, should lead to a gradual, albeit much delayed, remediation of groundwater.
Health significance
The reason for health risks lies in the danger of a reduction of nitrate to nitrite and the formation of carcinogenic nitrosamines. Such a conversion takes place on the one hand in the intestine by corresponding bacteria, on the other hand the salivary glands can also reduce nitrate washed up via the bloodstream. The intestinal flora of the infant (like the intestinal flora of an adult) may contain nitrite-forming bacteria. The resulting nitrite oxidizes hemoglobin to methemoglobin, which the infant cannot reduce back to hemoglobin because of its immature reduction capacity, so the infant suffocates from within. In ruminants, too, there is an acute danger of nitrite formation due to nitrate reduction in the rumen. Here, even nitrate concentrations above 20 mg/l in drinking water are considered harmful.
Nitrates in drinking water
The current limit value for NO3- in drinking water is 50 mg/l according to the German Drinking Water Ordinance. In Switzerland, a maximum value of 40 mg/l applies. In Austria, the limit value for nitrate according to the Drinking Water Ordinance is also 50 mg/l, but here the condition [NO3-]/50 + [NO2-]/3 ≤ 1 must be met (the values in the square brackets are calculated with concentrations in mg/l). Waters exceeding this limit are often mixed with lower nitrate water by water suppliers to comply with the limit. Recently, the first water treatment plants with reverse osmosis or nanofiltration have been built to lower the nitrate value in drinking water by partial desalination.
The higher the nitrate level, the higher the risk of colon cancer. An increase in risk can already be observed at nitrate concentrations that are significantly below the legally prescribed limits. There is a risk for infants and for people with degenerated intestinal flora due to the possible formation of nitrite. In the main, however, the limit values for nitrate serve as indicator values for a general contamination of drinking water sources with nitrogenous organic pollutants, which should remain contained (see paragraph on the philosophy of the limit values in the Drinking Water Ordinance). In 2014, the EU initiated infringement proceedings against Germany for excessive nitrate levels in groundwater. A further procedure is being prepared.
At the end of 2015, the German Advisory Council on the Environment (SRU) called for the development of a national nitrogen strategy, arguing that 27 % of all groundwater bodies are in poor chemical condition due to excessive nitrate content. Nitrate in the fermentation residues of biogas plants can also pollute groundwater if the quantities applied exceed plant requirements. Therefore, digestate should be separated and nutrients exported from the region if quantities of manure and digestate produced exceed nutrient needs in the region. With the aim of reducing nitrate levels in groundwater and thus averting EU fines, the Fertiliser Ordinance was tightened in Germany in 2017. In some regions, the nitrate limits are still being exceeded, so that Germany must act again. In Switzerland, too, the situation has hardly improved and the limit values are still not always complied with.
Saltpeter
Saltpetre, probably formed by dissimilation from Middle High German/Middle Latin salniter (designation for purified saltpetre), composed of Latin sal, 'salt', and Egyptian nṯr(.j), 'natron'. Kinship then exists with Hebrew neter and German 'Natron'. The German Dictionary of the Brothers Grimm traces the interpretation to Latin sal petrae, 'rock salt', 'rock salt'. Saltpetre is the trivial name of some frequently occurring nitrates. A distinction is made between the following types of saltpetre:
- Ammonium nitrate, combustible saltpetre (ammonium nitrate)
- Barytes nitrate (barium nitrate)
- Chile saltpetre, sodium nitrate (sodium nitrate)
- Bengal nitrate, potassium nitrate (potassium nitrate)
- Calcium nitrate, masonry nitrate (calcium nitrate)
In nature, they can be formed in different ways. Saltpetre is formed in arid, hot, vegetation-free areas during the biochemical decomposition (oxidation) of nitrogenous organic substances (guano and other excrement from birds and other animals), as well as from microalgae, nitrogen bacteria and others.
Furthermore, it can be formed by atmospheric nitrogen fixation and the corrosive action of nitric acid on rock debris of the desert floor. Nitrates are formed from tuffs in extensive liparite formations.
In earlier times, the producers of saltpetre were called saltpetre boilers. Historically, potash and Chile saltpetre are not only important as oxygen donors in blasting saltpetre (black powder). Saltpeter was the only source of large quantities of nitrogen compounds, especially nitrate fertilizer and nitric acid, until the discovery of the Haber-Bosch process for producing ammonia. It was shipped worldwide (see saltpetre voyage). As a result, conflicts arose over this raw material until the 20th century, e.g. the Saltpetre War in South America from 1879 to 1884. Several peasant uprisings in the Hotzenwald region led to the saltpetre riots in the 18th and 19th centuries. The Saltpetre Promise, a contract between Carl Bosch and the Supreme Army Command in 1914, was intended to enable the synthetic production of saltpetre on an industrial scale.
Evidence
The reaction with sulfuric acid and salicylic acid leads to an intensely yellow reaction product which can be used for a sensitive determination of the nitrate content in water samples according to DIN 38405-29 (German Standard Method D29), modified according to ISO 7890-3.
Qualitative detection methods are:
- Ring test
- Reduction with Devarda alloy to nitrite and subsequent detection with Lunges reagent
- qualitatively and quantitatively with the reagent according to Busch
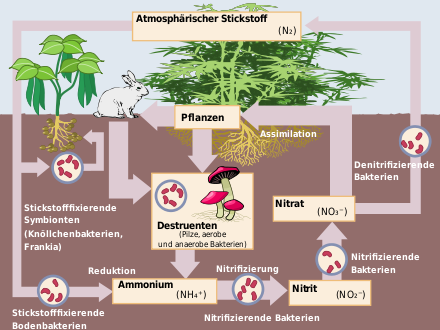
The nitrogen cycle

Calcium nitrate with 14,4 % nitrate nitrogen
Nitric acid ester
| Esters of nitric acid | ||
| Name | Formula | |
| Ethyl nitrate |
| |
| Glycerol trinitrate |
| |
| Cellulose nitrate |
| |
| For further examples see category:Nitric acid esters | ||
Organic nitrates - the nitric acid esters - are compounds which may decompose explosively. For this reason, the esters of polyalcohols are used as explosives (e.g. nitropenta, pentaerythritol trinitrate or glycerol trinitrate). Cellulose nitrate with varying degrees of nitration is used in pyrotechnics and is a constituent of nitrocellulose lacquers and celluloid.
Synthesis
Esters of nitric acid are obtained by esterification of an alcohol with concentrated nitric acid. The formation is spontaneous and weakly exothermic. It requires only some concentrated sulfuric acid as a catalyst.

Table tennis balls made of celluloid - a nitric acid ester
Search within the encyclopedia







