Nanotechnology
![]()
This article deals with nanoparticles in terms of their (technical) applications. For nanotechnology at the molecular level, see Molecular nanotechnology.
The collective term nanotechnology, often also nanotechnology (ancient Greek νᾶνος nános 'dwarf'), is based on the same order of magnitude of nanoparticles underlying all nano-research fields, from the single atom to a structural size of 100 nanometres (nm): one nanometre is one billionth of a metre (10-9 m). This order of magnitude denotes a borderline range in which the surface properties play an increasingly important role compared to the volume properties of the materials, and quantum physical effects must increasingly be taken into account. In nanotechnology, we are thus advancing to length scales where size in particular determines the properties of an object. One speaks of "size-induced functionalities".
Today, the term is used to describe the corresponding research in cluster, semiconductor and surface physics, surface and other areas of chemistry, as well as in sub-areas of mechanical engineering and food technology (nano-food).
Nanomaterials already play an important role today. They are mostly produced by chemical means or by mechanical methods. Some of them are commercially available and are used in commercially available products, others are important model systems for physico-chemical and materials science research.
Nanoelectronics is also important. Its classification as nanotechnology is not viewed uniformly in scientific and research policy practice. In many areas, the effects and influence of the mostly artificially produced particles on the environment are unclear and unexplored.
One direction of development of nanotechnology can be seen as a continuation and extension of microtechnology (top-down approach), but a further reduction in the size of micrometer structures usually requires completely unconventional new approaches. Chemistry often follows the opposite approach in nanotechnology: bottom-up. Chemists who usually work in molecular, i.e. sub-nanometer dimensions, build larger nanoscale molecular assemblies from a large number of individual molecular units. Dendrimers are one example of this.
A small branch of nanotechnology deals with nanomachines (see molecular machine) or nanobots.
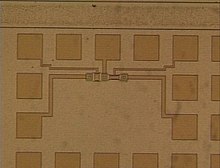
Already today the size of the transistors (see picture) of a commercial microprocessor is in the range of nanotechnology. Structures 5 nm wide are achieved.
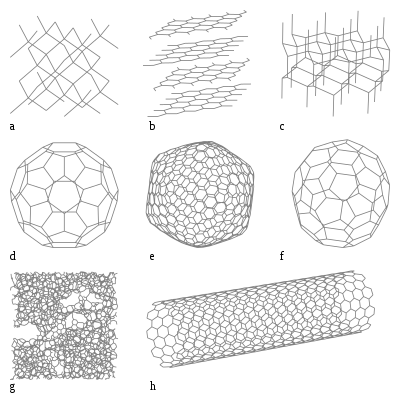
New materials such as fullerenes (d-f) or carbon nanotubes (h) are nanotechnology and are already used in many fields
Origins of nanotechnology
Richard Feynman is considered to be the father of nanotechnology because of his lecture "There's Plenty of Room at the Bottom", held in 1959, although it was Norio Taniguchi who first used the term "nanotechnology" in 1974:
"Nano-technology mainly consists of the processing of separation, consolidation, and deformation of materials by one atom or one molecule."
Nanotechnology in the sense of this definition is the modification of materials, whether atom by atom or molecule by molecule. This includes the fact that the critical properties of materials or devices can be on the nanometer scale, and that these materials and devices are constructed from individual atoms or molecules. Today, however, nanotechnology is rarely used in this narrow sense; today, the term also includes (as explained above) the production of nanomaterials by chemical means.
Independently of Taniguchi, Eric Drexler made the term widely known in 1986. With his book Engines of Creation, he inspired many scientists and physicians known today, including Richard E. Smalley (Fullerene), to study nanotechnology. Drexler's definition of nanotechnology is stricter than Taniguchi's: it is limited to the construction of complex machines and materials from individual atoms.
According to this definition, today's nanotechnology does not fall under what Drexler regards as nanotechnology. In the course of the 1990s, this led Drexler to rename his concept of nanotechnology Molecular Nanotechnology (MNT) in order to distinguish it from other nanotechnologies, because the term was and is often used to describe all work dealing with nanostructures, even if ordinary chemical, pharmaceutical or physical methods are used.
Indeed, many scientists are currently skeptical to openly hostile to Drexler's vision of nanotechnology. Even if, according to the proponents of MNT, their opponents have not yet succeeded in presenting convincing scientific arguments against the feasibility of MNT, many still consider its feasibility to be unlikely; even though Drexler published Nanosystems, a textbook on MNT in 1991, which, based on his doctoral thesis at the Massachusetts Institute of Technology (MIT), describes in scientific form the steps necessary for its realization. Over the years, some of Drexler's assumptions have been experimentally confirmed, but many caveats remain that stand in the way of realization: Even if it were possible to make a nanomotor out of metal, for example, it would not be functional for long: the very film of water that forms on the metal surface due to the condensation of atmospheric moisture would paralyze the motor. Metals such as iron, steel or aluminium form a thin oxide film in air, which does not interfere with ordinary workpieces. However, oxidation of nanometals usually results in complete conversion to the oxide. A nanomotor made of metal would therefore be virtually incinerated by atmospheric oxygen. So you could only build an engine that is made of a substance that does not oxidize by water. If you wanted to move macromolecules past each other in vacuum or in air at a distance of less than a few atomic diameters, they would stick together due to Van der Waals forces. But if you embed the macromolecules in water or some other suitable liquid, then the liquid takes over the Van der Waals forces, and you can move the macromolecules past each other with little friction. This is how living cells work, and the flagellar drive of bacteria reaches 50 revolutions per second. Holding or releasing individual atoms or molecules purely mechanically is also complicated by Van der Waals forces, which has been called the 'sticky finger problem'. This problem, and also the purely mechanical creation of covalent bonds, was overcome by applying an electrical voltage, which was demonstrated here.
An example of the use of nanotechnology in the 4th century AD is the Lykurgos cup. The optical dichroic effect could not be explained at the time, but is based on nanoparticles of gold and silver dispersed in the glass. The manufacturing process is still not fully understood today.

The Lycurgus Cup, 4th century dichroic Roman glass with nanoparticles.
Models in nature
Effects like those used by many nanotechnologies often occur in nature. For example, there are nanometre-sized hairs on the legs of flies, which are the reason why these insects can walk on ceilings and walls. The best-known example of nanotechnology is the lotus effect: fine nanostructures ensure that water beads off the leaf of the lotus flower, minimizing the adhesion of dirt particles. The wings of the glass-winged butterfly appear transparent and reflect only a fraction of infrared to ultraviolet radiation due to irregular nanocolumns. Also, in the lime of mussel shells, organic and inorganic substances are so closely aligned on the nanoscale that mussel shells are extremely stable and resistant, the same effect exists in human bone. Furthermore, a large number of nanoparticles are released in every combustion. The enzyme molecules, the ribosomes, and the flagellar drives of bacteria mentioned above are also natural nanomachines.
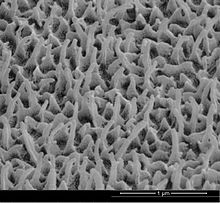
Nanopillars on the wing surface of the glass wing butterfly

Lotus effect on lotus sheet
Questions and Answers
Q: What is nanotechnology?
A: Nanotechnology is a part of science and technology about the control of matter on the atomic and molecular scale, which includes making products that use parts this small, such as electronic devices, catalysts, sensors, etc.
Q: How small are nanometres?
A: Nanometres are incredibly small - there are more nanometres in an inch than there are inches in 400 miles. To give an international idea of how small that is, there are as many nanometres in a centimetre, as there are centimetres in 100 kilometres.
Q: What types of work do people do in the field of nanotechnology?
A: People working in the field of nanotechnology look at making nanoparticles (particles with nanometer size) that have special properties like scattering light or absorbing X-rays. They also attempt to make small copies of bigger machines or really new ideas for structures that make themselves. New materials can be made with nano size structures and it's even possible to work with single atoms.
Q: What potential applications does nanotechnology have?
A: Nanotechnology has potential applications across many different fields including medicine, computers and clean electricity production (nanoelectromechanical systems). It could also help design next generation solar panels and efficient low-energy lighting.
Q: Are there any risks associated with using nanotechnology?
A: There could be unknown problems associated with using nanotechnology such as if the materials used were bad for people's health or for nature. They may have a bad effect on the economy or even big natural systems like the Earth itself so some groups argue that rules should be put into place regarding its use.
Q: What type of scientists study nano technology?
A: Scientists studying nano technology come from many different disciplines including applied physics, materials science, interface and colloid science, device physics, chemistry supramolecular chemistry self-replicating machines and robotics chemical engineering mechanical engineering biology biological engineering electrical engineering etc
Search within the encyclopedia