Molar mass
The molar mass 



It is an intensive quantity. The SI unit is kg/mol; in chemistry g/mol is common.
The molar mass of a chemical compound is the sum of the molar masses of the chemical elements involved in the compound multiplied by the respective stoichiometry factor. Stoichiometry factors are the numbers in the molecular formula of a molecule or, in the case of non-molecular compounds (metals and ionic compounds), the ratio formula.
The molar mass of an isotopically pure substance is constant. The numerical value of the molar mass in g/mol is the relative molecular mass and is equal to the numerical value of the molecular mass in the atomic mass unit (u or dalton if the compound consists of nuclide-pure elements).
Definition
The individual formula symbols stand for the following quantities:
- mass
- amount of substance
- Avogadro constant
- particle mass
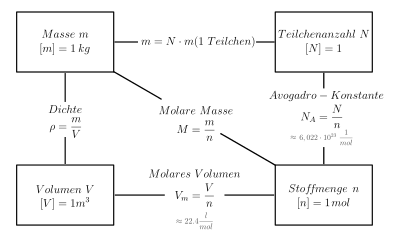
The relationship between mass, amount of substance, volume and number of particles.
Calculation
The molar mass of a compound can be calculated if its molecular formula is known: For each element, the index number is taken from the molecular formula - it is given in the molecular formula after the element symbol. For each element, the molar mass must then be taken from tables, for example - its numerical value is equal to the relative atomic mass. Then you get the molar mass as the sum of the molar masses of the elements that make up the compound:
The molar mass of a compound is equal to the sum of the molar masses of the elements multiplied by their index numbers.
Example of water (H2O):
From the molar masses of the chemical elements one can calculate the molar masses of all compounds.
| Item | Element symbol | Atomic number | Molar mass |
| Hydrogen | H | 1 | 1.00794 g/mol |
| Carbon | C | 6 | 12.0107 g/mol |
| Oxygen | O | 8 | 15.9994 g/mol |
| Link | Sum formula | atomicity | Molar mass |
| Hydrogen | H2 | 2 | 2.01588 g/mol |
| Oxygen | O2 | 2 | 31.9988 g/mol |
| Water | H2O | 3 | 18.01528 g/mol |
| Methane | CH4 | 5 | 16.043 g/mol |
| Acetylsalicylic acid | C9H8O4 | 21 | 180.16 g/mol |
Search within the encyclopedia

