Methane
Methane is a chemical compound with the molecular formula CH4 and the simplest representative of the alkanes. Under normal conditions it is a colourless and odourless flammable gas. Methane is insoluble in water and forms explosive mixtures with air. It burns with a bluish-bright flame in the presence of sufficient oxygen to form carbon dioxide and water.
It occurs naturally and is the main component of natural gas. Since it occurs in large quantities in deposits, it is an attractive source of energy. It is transported by pipelines or as a deep-frozen liquid by tankers. It also occurs as methane hydrate bound to the sea floor and permafrost areas, although the exact supply is unknown. Methane is used as a heating gas and is of great importance in the chemical industry as a starting material for technical syntheses such as methanol production or the production of halogenated methane derivatives. It is also used for the production of hydrogen on a large industrial scale.
The gas is produced in considerable quantities by biological processes, either anaerobically by microorganisms or aerobically by phytoplankton, plants and fungi. Anthropogenic sources include rice cultivation and cattle breeding. Abiotic sources such as forest fires or volcanic eruptions also release methane.
Methane is the most abundant hydrocarbon in the terrestrial atmosphere, with concentrations varying both between the northern and southern hemispheres and seasonally. As a greenhouse gas, methane has a high global warming potential. It contributed to global warming in climate history and is influencing current global warming. In the Earth's atmosphere, it is oxidized to water, formaldehyde, and eventually carbon dioxide. Methane is a component of the atmospheres of other planets and moons and has been detected in comets as well as in the interstellar medium.
History
Before there was an understanding of what methane was, it appeared as a component of natural gas-fed "eternal fires", for example in Chimaira (Lycia). These fires have been known since as early as 2000 BC. The eternal fire at Baba Gurgur in Iraq may be the furnace described in the Bible in the book of Daniel, where King Nebuchadnezzar threw Daniel's companions Shadrach, Meshach, and Abednego for refusing to worship a golden statue (Dan 3:23 EU).
Methane as a component of natural gas was already used energetically in China in the 4th century BC for the evaporation of brine. In part, both methane and brine escaped from the boreholes, in part the gas was brought in by means of bamboo pipelines from so-called dry boreholes for brine processing.
Nothing was known about the nature of the gas at that time, but it was known to alchemists in the Middle Ages as a component of putrid gases, also known as "swamp air". In February 1659, Thomas Shirley, an Englishman, investigated a spring of flammable water near Wigan. He was able to show that it was not the water but a gas rising from the bottom of the impounded water that caused the phenomenon. He suspected that the gas came from an underlying coal deposit and that it was mine gas. The occurrence of firedamp, a gas mixture of methane and air, was known to be a hazard in underground mining early on because an ignition source could trigger a firedamp explosion.
The Italian physicist Alessandro Volta discovered methane in the swamps of Lake Maggiore in 1776, inspired by an essay by Benjamin Franklin on "flammable air". Volta collected the gas rising from the swamp and isolated the pure gas by 1778. Marcellin Berthelot produced methane for the first time in 1856 from carbon disulfide and hydrogen sulfide.
The name methane is derived from Méthylène. This was the first name given in 1834 by the French chemists Jean-Baptiste Dumas and Eugène-Melchior Péligot to the liquid now known as methanol. Its name is composed of ancient Greek méthy (ancient Greek μέθυ) for intoxicating drink or wine and hýlē (ancient Greek ὕλη) for wood. The German chemist August Wilhelm von Hofmann first proposed a systematic nomenclature in an English publication in 1866, according to which the name for the gas derived from methylene and should be called methanes. This gave rise to the German form methane. In old texts, methane was occasionally referred to as methyl hydrogen.
With the start of oil production, methane was mostly flared at the wellhead as the main component of the natural gas produced at the same time. As global energy demand increased, methane became a steadily growing factor in world primary energy production. Between 1980 and 1990, its share rose from about 18% to about 22%.

Alessandro Volta
Occurrence
Earthly
Methane, along with ammonia and water vapor, was a major component of the Earth's primordial atmosphere. It occurs in a variety of forms and is constantly being formed on Earth, for example in technical, biological or geological processes such as serpentinisation. The concentration of methane in the Earth's atmosphere more than doubled from 0.73 to 1.869 ppm between 1750 and 2018, reaching its highest level in 800,000 years.
In the atmosphere, methane first reacts with oxygen to form formaldehyde and possibly ozone. Further oxidation eventually produces carbon dioxide.
Near Surface
Microorganisms form a large part of terrestrial methane. When organic matter rots in the absence of air in swamps or in the sediment at the bottom of bodies of water, swamp gas forms, a mixture of methane and carbon dioxide. Biogas contains about 60% methane and about 35% carbon dioxide, along with smaller amounts of hydrogen, nitrogen, and hydrogen sulfide.
The formation takes place biotically near the surface: on the one hand, anaerobically in the course of methanogenesis from carbon dioxide and acetate by a group of archaea, the methanogens. They use simple compounds such as carbon dioxide or methanol and reduce them to methane, gaining energy in the process. For example, the formation of methane from CO2 and hydrogen (H2) under standard conditions at a pH of seven releases about 131 kJ/mol of free enthalpy (Gibbs energy, ΔG0'):
A small part of the biotic formation is based on the aerobic cleavage of methyl phosphonates.
Underground
Below the surface of the earth, methane is formed in the deeper subsurface at high temperatures and pressures and is usually released during volcanic activity. As a component of natural gas, its concentration varies between 35 and 98 % depending on the deposit. Other natural gas constituents may include higher hydrocarbons, especially ethane, as well as carbon dioxide and nitrogen. "Wet" natural gas, which usually occurs together with crude oil, contains hydrocarbons such as pentane, hexane, heptane and even higher hydrocarbons, while "dry" natural gas contains methane as its main component. Mine gas trapped in hard coal deposits contains mainly methane.
It can be formed abiotically thermally as part of the maturation process of coal in the geochemical phase of coalification, as well as from all types of kerogens and petroleum. In 2008, methane from the Lost City hydrothermal vents was shown to be of geochemical origin.
Methane hydrate
Methane seeping from the ocean floor reacts with water under high pressure and low temperature to form solid methane hydrate, also known as "methane ice". The carbon content of the world's methane hydrate deposits was estimated to be about 11,000 gigatonnes in the late 1980s, and the carbon content of proven natural gas, coal and oil reserves is about 5000 gigatonnes. More recent estimates suggest much lower reserves, with estimated reserves of about 1800 gigatonnes of carbon.
Alien
| Methane content in planetary atmospheres | |
| Planet | Share |
| Earth | 1.86 ppm |
| Mars | 0.0105 ppm |
| Jupiter | 2000 ppm |
| Saturn | 4500 ± 2000 ppm |
| Uranus | 20-40,000 ppm |
| Neptune | 15,000 ± 5000 ppm |
The atmospheres of the planets Mars, Jupiter, Saturn, Uranus, Neptune and the dwarf planet Pluto also contain methane, as do the atmospheres of moons such as Titan or Enceladus. After hydrogen and helium, methane is the third most abundant component of Uranus' atmosphere. Because of its absorption bands in the red and near-infrared, Uranus appears aquamarine or cyan. Saturn's slightly lighter blue hues are probably due to the same effect.
Outside our solar system, methane has been detected as the first organic molecule on the exoplanet HD 189733 b of the Hot Jupiter type. The absorption spectra of cool brown dwarfs, called methane T-dwarfs, are dominated by methane and water. In addition, there are hotter, so-called L dwarfs, which also have methane absorption features, but these are weaker than those of the T dwarfs.
Mars
In 2009, methane eruptions were reported on Mars; methane was detected in the Martian atmosphere, about 10.5 ppb. Since it cannot normally persist in the atmosphere and there is no evidence of meteorites as a source, it must have been newly formed on the planet, which could be an indication of life on Mars. The methane could be of volcanic origin, for which no evidence has yet been found on Mars.
Titanium
See also: Methane lakes on Titan
On Saturn's moon Titan, at -180 °C and about 1.6 bar atmospheric pressure, the triple point of methane is almost reached. Methane can therefore occur in all three states of aggregation on this moon. There are clouds of methane, from which methane rains, which flows through rivers, including the Vid Flumina, into methane lakes, where it evaporates again, forming a closed methane cycle, analogous to the water cycle on Earth. Liquid methane is transparent to radar beams, which is how the Cassini space probe was able to determine the depth of the Ligeia Mare lake to be 170 metres.
There are probably icebergs of methane and ethane on these lakes. These float on the methane lakes when they contain at least 5% gaseous nitrogen. If the temperature drops only slightly, the nitrogen contracts to such an extent that the ice sinks to the bottom. When the temperature rises again, the ground ice can rise back to the lake surface. At certain temperatures, surface and bottom ice can occur simultaneously. For Ontario Lacus, a lake near Titan's south pole, ethane has been found to be the main constituent.

Methanometer of the Zollern colliery
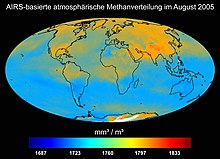
Atmospheric methane distribution in August 2005, measured with the Atmospheric Infrared Sounder (AIRS) on board the Aqua satellite.

Mercator projection of Saturn's moon Titan, taken by the Huygens lander at an altitude of 10 kilometres. In the upper, central area, structures can be seen that may have originated from methane flows (14 January 2005).
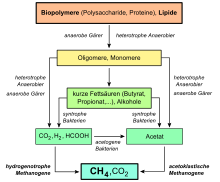
Overview of the anaerobic utilization of polymeric substrates and lipids
Questions and Answers
Q: What is methane?
A: Methane is an organic compound with the chemical formula CH4.
Q: What type of compound is methane?
A: Methane is an alkane with one carbon atom.
Q: Where is methane often found?
A: Methane is often found as the main part of natural gas.
Q: How effective is methane compared to carbon dioxide as a greenhouse gas?
A: Methane is a greenhouse gas 23 times more effective than carbon dioxide.
Q: What happens to methane when it oxidizes with oxygen?
A: Methane slowly oxidizes by oxygen to carbon dioxide and water.
Q: Is methane stable?
A: Methane is less stable than some compounds.
Q: What is the chemical formula for methane?
A: The chemical formula for methane is CH4.
Search within the encyclopedia


