Metastability
![]()
This article is about the thermodynamic meaning. For other meanings, see Metastability (disambiguation).
Metastability is a weak form of stability. A metastable state is stable against small changes but unstable against larger changes.
An example of this is the system of wood and atmospheric oxygen at room temperature: from a thermodynamic point of view, the spontaneous combustion of the carbon chemically bound in it with the oxygen to form carbon dioxide would lead to a more stable state. However, this will not happen without activation, i.e. a sufficiently large energy input such as igniting the wood.
This is clearly illustrated in the picture on the right: A ball lies in a small hollow on a mountain slope. As long as the ball is only slightly deflected in the hollow, it rolls back to its deepest point. This represents a local minimum. But if it is deflected more, it can roll down the hillside and reach the global minimum. So first a certain minimum energy must be applied before the state of the system changes.
Metastable phases have a higher energy (more correctly: free enthalpy - under defined conditions such as constant pressure and constant temperature) than the stable phase. Due to a high activation energy, they do not transform into the stable phase or only slowly. This basic energy principle of metastable states can also be used as a method of energy storage, as is also done in principle in storage power plants, for example.
An example of a metastable phase is diamond, which should spontaneously transform into graphite at atmospheric pressure; however, the speed of this process is vanishingly small at room temperature. Another example is tin plague: the metallic phase of tin becomes metastable below 13 °C and slowly transforms into the nonmetallic phase, which is more stable at these temperatures. Other examples are supercooled water, glass (the most stable state would be the crystalline silicates) and supersaturated solutions, which are used in hand warmers, for example.
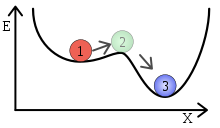
A metastable system: state 1 is stable to small perturbations and transitions to state 3 in the presence of large perturbations. State 2 is unstable.

Many natural systems are metastable, so burns only occur after sufficient activation.

An unstable system leaves its initial state after an infinitesimal perturbation and does not return.
See also
- Bistability
Questions and Answers
Q: What is metastability?
A: Metastability is a state in which something is not changing but can transition to another, more stable state given a small force.
Q: Can you give an example of metastability?
A: Yes, a block sitting on a slope is an example of something that is metastable. It remains in place until it is nudged, at which point it transitions to a more stable state by sliding down to the bottom of the slope.
Q: How does something become unstable?
A: Something becomes unstable when it is in motion, such as when the block on a slope starts to slide down after being nudged.
Q: What is an example of something that is unstable?
A: An avalanche of snow is an example of something that is unstable, as the snow is sliding down the mountainside.
Q: What is a real-life example of metastability?
A: A real-life example of metastability is the snow on a mountainside before an avalanche. The snow is in a state of metastability, as it can be easily triggered into an unstable state by a small perturbation.
Q: How does the block on a slope illustrate the concept of metastability?
A: The block on a slope is an example of metastability because it appears stable but can transition to a more stable state (at the bottom of the slope) if nudged.
Q: What is the general idea behind metastability?
A: The general idea behind metastability is that something is in a state of apparent stability but is actually only temporarily stable and can transition to a more stable state given a small force.
Search within the encyclopedia