Metal
![]()
Metal is a redirect to this article. For other meanings, see Metal (disambiguation).
· 
Crystal of 99.999 % pure gallium
· 
15 cm tall gold nugget (National Museum of Natural History)
·
Copper disc produced by the continuous casting method, etched, purity ≥99.95 %, Ø≈10 cm.
Metals (from the ancient Greek μέταλλον metallon "mine, ore, metal") constitute those chemical elements that are to the left of and below a dividing line from boron to astatine in the periodic table of elements. This is about 80 percent of the chemical elements, with a smooth transition to the nonmetals via the semimetals, many of which can form modifications with metallic and atomic bonding.
The term is also used for alloys and some intermetallic phases; it applies to all materials which, in solid or liquid form, exhibit the following four characteristic metallic material properties:
- high electrical conductivity, which decreases with increasing temperature,
- high thermal conductivity,
- Ductility (deformability)
- metallic sheen (mirror finish).
All these properties are based on the fact that the cohesion of the atoms in question takes place with the metallic bond, whose most important feature is the freely moving electrons in the lattice.
A single atom of these elements has no metallic properties; it is not a metal. Only when several such atoms interact with each other and a metallic bond exists between them do such groups of atoms (clusters) exhibit metallic properties.
Atoms of these elements can also assemble amorphously when cooled extremely quickly without forming a crystal lattice - see metallic glass.
On the other hand, atoms of other elements can also form metallic bonds under extreme conditions (pressure) and thus take on the metallic properties mentioned - see metallic hydrogen.
Since the beginning of civilization, metals have been used as materials in a variety of applications. Under the term metal physics or also metallurgy, physicists and materials scientists deal with all the fundamentals, see under solid state physics, and with applications, see under materials science.
Division
| The elements divided into nonmetals, semimetals and metals. The latter are differentiated according to density (calculated for Fm to Og) | ||||||||||||||||||
| H | He | |||||||||||||||||
| Li | Be | B | C | N | O | F | Ne | |||||||||||
| Na | Mg | Al | Si | P | S | Cl | Ar | |||||||||||
| K | Ca | Sc | Ti | V | Cr | Mn | Fe | Co | Ni | Cu | Zn | Ga | Ge | As | Se | Br | Kr | |
| Rb | Sr | Y | Zr | Nb | Mon | Tc | Ru | Rh | Pd | Ag | Cd | In | Sn | Sb | Te | I | Xe | |
| Cs | Ba | La | * | Hf | Ta | W | Re | Os | Ir | Pt | Au | Hg | Tl | Pb | Bi | Po | At | Rn |
| Fri | Ra | Ac | ** | Rf | Db | Sg | Bh | Hs | Mt | Ds | Rg | Cn | Nh | Fl | Mc | Lv | Ts | Og |
|
| ||||||||||||||||||
|
| * | Ce | Pr | Nd | Pm | Sm | Eu | Gd | Tb | Dy | Ho | He | Tm | Yb | Lu | |||
|
| ** | Th | Pa | U | Np | Pu | At | Cm | Bk | Cf | It | Fm | Md | No | Lr | |||
Traditionally, metals are divided according to density into heavy metals and light metals and according to reactivity into noble metals and base metals, the latter being good reducing agents. See also the main article Metallic material (and on reactivity under Redox reaction).
Metals are formed by the elements that are located in the periodic table of the elements in the area to the left and below a line from boron to astatine, whereby the metallic character increases from top to bottom or from right to left. At the very top right are the nonmetals, with the semimetals in between. The subgroup elements form metals without exception. The border to the non-metals is fluid. For example, antimony, arsenic, cerium, and tin have both metallic and nonmetallic modifications.
The affiliation to main or subgroups of the periodic table is also decisive for the chemical behaviour.
See also: Refractory metals
Physical properties
General
Prerequisite for the formation of the metallic state are the following properties of atoms:
- The number of electrons in the outer shell is small and smaller than the coordination number
- The ionization energy (necessary to split off these outer electrons) is small (< 10 eV).
As a result, such atoms cannot bond with each other via atomic bonds to form molecules or lattices. At most, atomic bonds occur in metal vapours, e.g. sodium vapour consists of about 1 % Na2 molecules.
Rather, metal atoms arrange themselves into a metal lattice consisting of positively charged atomic trunks, while the valence electrons are distributed throughout the lattice; none of these electrons belongs to a particular nucleus anymore. These freely moving electrons can be thought of as particles of a gas that fills the space between the atomic trunks. Since this electron gas, among other things, causes the good electrical conductivity of metals, the energy level at which the free electrons are found is called the "conduction band." The exact energetic conditions are described by the band model based on the orbital model.
This type of bonding and this lattice structure result in the following typical properties of the metals:
- Shine (mirror shine): The freely moving electrons can re-emit almost all of the incident electromagnetic radiation up to wavelengths of X-rays; this is how the shine and reflection are created; mirrors are therefore made from smooth metal surfaces.
- Opacity: The aforementioned reflection taking place on the metal surface and the absorption of the non-reflected portion have the effect that, for example, light can hardly enter metal. Metals are therefore only somewhat translucent in the thinnest layers and appear grey or blue when seen through.
- Good electrical conductivity: The migration of freely moving electrons in one direction is the electric current.
- Good thermal conductivity: The easily displaceable electrons participate in the thermal motion. They also transfer the inherent thermal motion of the atomic hulls (oscillations) and thus contribute to heat transport, cf. heat conduction.
- Good formability (ductility): there are grain boundaries and dislocations in the metal which can already move at an elongation below the breaking elongation, i.e. without losing cohesion; depending on the type of lattice, a metal therefore deforms before it breaks.
- Relatively high melting point: It results from the omnidirectional bonding forces between the cations and the freely moving electrons, but a less strong effect than the electrostatic attraction forces between ions in salt crystals.
Melting and boiling temperatures
Metals whose melting point TE is above 2000 K or above the melting point of platinum (TE platinum = 2045 K = 1772 °C) are described as having a high melting point. These include the precious metals ruthenium, rhodium, osmium and iridium and metals of groups IVB (zirconium, hafnium), VB (vanadium, niobium, tantalum), VIB (chromium, molybdenum, tungsten) and VIIB (technetium, rhenium).
Heat conduction properties
The properties relevant to thermal conduction, such as density, heat capacity, thermal conductivity and thermal diffusivity, vary greatly. Silver, for example, has a thermal conductivity of 427 W/(m-K), which is about 50 times higher than manganese, see list of values.
| Physical properties of some metals. The highest and lowest values are marked in colour. | ||||||||||||||
| Item | Lithium | Aluminium | Chrome | Iron | Copper | Zinc | Silver | Tin | Caesium | Wolfram | Osmium | Gold | Mercury | Lead |
| Melting point in °C (1013 hPa) | 180,54 | 660,2 | 1907 | 1538 | 1084,62 | 419,53 | 961,78 | 231,93 | 28,44 | 3422 | 3130 | 1064,18 | −38,83 | 327,43 |
| Boiling point in °C (1013 hPa) | 1330 | 2470 | 2482 | 3000 | 2595 | 907 | 2210 | 2602 | 690 | 5930 | 5000 | 2970 | 357 | 1744 |
| Density in g/cm3 (20 °C, 1013 hPa) | 0,534 | 2,6989 | 7,14 | 7,874 | 8,92 | 7,14 | 10,49 | α-Tin: 5,769 β-Tin: 7,265 | 1,90 | 19,25 | 22,59 | 19,32 | 13,5459 | 11,342 |
| Mohs hardness | 0,6 | 2,75 | 8,5 | 4,0 | 3,0 | 2,5 | 2,5 | 1,5 | 0,2 | 7,5 | 7,0 | 2,5 | 1,5 | |
| Electrical conductivity in 106 S/m | 10,6 | 37,7 | 7,87 | 10,0 | 58,1 | 16,7 | 61,35 | 8,69 | 4,76 | 18,52 | 10,9 | 45,5 | 1,04 | 4,76 |
| Thermal conductivity in W/(m-K) | 85 | 235 | 94 | 80 | 400 | 120 | 430 | 67 | 36 | 170 | 88 | 320 | 8,3 | 35 |
| Atomic number | 3 | 13 | 24 | 26 | 29 | 30 | 47 | 50 | 55 | 74 | 76 | 79 | 80 | 82 |
| atomic mass in u | 6,94 | 26,982 | 51,996 | 55,845 | 63,546 | 65,38 | 107,868 | 118,710 | 132,905 | 183,84 | 190,23 | 196,967 | 200,592 | 207,2 |
| Electronegativity | 0,98 | 1,61 | 1,66 | 1,83 | 1,9 | 1,65 | 1,93 | 1,96 | 0,79 | 2,36 | 2,2 | 2,54 | 2,0 | 2,33 |
| Crystal system(1) | cl | cl | cl | cl | cF | hcp | cF | α-tin: A4 β-Tin: tl | cl | cl | hcp | cF | P3 | cF |
(1) cl: body-centered cubic, cF: face-centered cubic, hcp: hexagonal closest sphere packing, A4: diamond structure, tl: tetragonal inner-centered, P3: rhombohedral

A piece of high purity iron with 99.97% purity
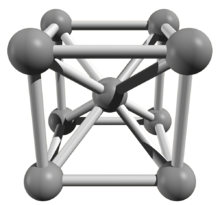
Cubic space centered elementary cell of an iron crystal
Questions and Answers
Q: What are some traits of elements that are classified as metals?
A: The traits of elements that are classified as metals include their ability to conduct electricity and heat, their ease of shaping, their shiny appearance, their high melting point, and their maleability.
Q: How many elements in the periodic table are classified as metals?
A: Most of the elements in the periodic table are classified as metals.
Q: Are all metals solid at room temperature?
A: No, not all metals are solid at room temperature. Mercury, for example, is a liquid.
Q: What are alloys?
A: Alloys are mixtures where at least one part of the mixture is a metal.
Q: Can you give some examples of metals?
A: Some examples of metals include aluminium, copper, iron, tin, gold, lead, silver, titanium, uranium, and zinc.
Q: What is metallurgy?
A: Metallurgy is the study of metals.
Q: What are some well-known alloys?
A: Some well-known alloys include bronze and steel.
Search within the encyclopedia
