Mercury battery
The mercury oxide zinc battery has an anode of zinc powder and a cathode of mercury oxide. The electrolytes form a jelly-like mass of zinc hydroxide. A mercury oxide zinc battery provides an extremely constant voltage of 1.35 volts. Towards the end of the battery's life cycle, the voltage drops very rapidly. For this reason, this type of cell was often used by the photographic industry for the operation of exposure meters, since this requires a reference voltage that is as constant as possible. The button cell type with the designation PX625 was preferred for this purpose.
When the battery is operated, the following reactions take place in a simplified manner:
Reduction:
oxidation:
The redox reaction can be described in abbreviated form as follows:
The overall response is as follows:
Mercury oxide zinc batteries in button cell form used to be used a lot in small devices with low power requirements (e.g. wrist watches) or as hearing aid batteries. However, these button cells pose a major problem for the environment if not disposed of properly. When they are used up, they contain mercury. If the cell is damaged, the mercury leaks out and contaminates the environment. In watches, they have been replaced by silver oxide zinc batteries. In hearing aids, on the other hand, they are replaced by zinc-air batteries, which have a higher energy density but also a higher self-discharge.
EU Directive 91/157/EEC banned batteries containing more than 25 mg of mercury and alkaline manganese batteries containing more than 0.025% mercury from 1992. In 1999, the ban was extended in such a way that all batteries may contain a maximum of 0.0005% of their filling weight in mercury. In Germany, this is regulated by the Battery Ordinance. A similar ban was passed in the USA in 1996 with the Mercury-Containing and Rechargeable Battery Management Act. In the meantime, the production and trade of and with mercury oxide zinc batteries has been discontinued worldwide.
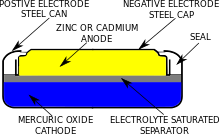
Structure of a cell

Soviet style mercury oxide zinc button cell (1989) with 0.32 Ah
Search within the encyclopedia



