Lone pair
A free electron pair (also called non-bonding or lone pair) consists of two electrons on an atom that have opposite spin and occupy the same atomic and molecular orbital. In chemistry, these electrons are also called valence electrons. As a pair of valence electrons, the free electron pair is generally not involved in any bonding with other atoms, but belongs only to one atom - an exception being, for example, ozone. In a valence line formula, a free electron pair is represented either by two dots (IUPAC recommendation) or by a line on the atom in question. There is also the representation of free electrons as electron clouds. The following figures show molecules with free electron pairs marked in blue:
· 
Water with two pairs of electrons as points.
· 
Water with two pairs of electrons as dashes.
· 
Water with two electron pairs as electron clouds.
· ![]()
Carbon dioxide with four free electron pairs.
· ![]()
Hydrogen cyanide with a free electron pair.
· 
Ammonia with a free electron pair.
· ![]()
Nitrogen molecule with two free electron pairs.
· ![]()
Nitrogen molecule with two electron clouds.
· 
Ozone with six free electron pairs.
· ![]()
Hydrogen chloride with three free electron pairs.
Free electron pairs contribute to the spatial structure of molecules, the shape of which can be predicted for simple compounds using the electron pair repulsion model (VSEPR model). The best-known example is the angled shape of the water molecule, which is crucial for some of the properties of water.
In contrast to a free electron pair, a binding electron pair represents the bond between two atoms. This is called a covalent bond.
Chirality due to a free electron pair.
A free electron pair behaves like another substituent at a stereocenter.
Amines
If a tertiary amine NR1R2R3 possesses three different organic radicals (R1 ≠ R2 ≠ R3) and a free electron pair at the nitrogen atom, one might expect such amines to be chiral. However, at room temperature, enantiomers cannot usually be isolated because of the rapid inversion ("swinging through" of the free electron pair). However, this does not apply to special tertiary amines in which the nitrogen atom is prevented from inversion by a "bridgehead" position (e.g., Tröger's base). Therefore, there are two stable enantiomers of the Tröger base, which can be separated chromatographically on a chiral stationary phase, for example.
Sulfoxides
Sulfoxides of the type O=SR1R2 are, in the case of two different organic residues (R1 ≠ R2), chiral due to the free electron pair at the sulfur atom.
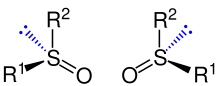
Sulfoxides are chiral if the radicals R1 and R2 are different. Shown is a pair of enantiomers of sulfoxides.
Questions and Answers
Q: What is a lone pair?
A: A lone pair is a group of two electrons that remain after bond formation and are not used in any bonds between atoms.
Q: Where are lone pairs located?
A: Lone pairs are always located in the valence shell of an atom.
Q: What is the function of lone pairs in bonding between molecules?
A: Lone pairs can be used to form new bonds between molecules.
Q: What is the energy level of lone pairs?
A: Lone pairs are usually high in energy.
Q: What is the role of a nucleophile regarding lone pairs?
A: Nucleophiles always have a lone pair that is used to attack an electrophile.
Q: How do lone pairs affect the shape of a molecule?
A: Lone pairs take up more space around an atom than a bonding electron, and lone pairs on the same atom want to be as far away from each other as possible, which affects the overall shape of a molecule.
Q: Do all atoms have lone pairs?
A: Not all atoms have lone pairs, but only those in the valence shell.
Search within the encyclopedia