Law of definite proportions
The law of constant proportions states that the elements in a given chemical compound always occur in the same mass ratio. For example, the mass ratio in sodium chloride is always 39% sodium to 61% chlorine.
This rule, formulated by Joseph-Louis Proust in 1797 and also called Proust's law after him, is the starting point for the development of stoichiometry. John Dalton extended it to the law of multiple proportions.
The law itself had already become common knowledge among practical chemists in the 18th century, and was referred to as a dogma of eternal truth in the French Encyclopedia of 1765, for example. Joseph Louis Proust undertook numerous experiments and analyses of minerals and oxides from 1797 to 1809 in which he found the law confirmed, and he formulated it in several publications, including one in 1797 and one in 1799. The otherwise tacitly assumed law did not receive greater attention from chemists until Claude Louis Berthollet criticized the law in his Essai de statique chimique, published in 1803. Berthollet held that arbitrary ratios occur within certain limits. Proust proved in the dispute that the oxides studied by Berthollet were mixtures of different oxides of the same metal, but each in a constant ratio of metal to oxygen. He thus anticipated the law of multiple proportions generally attributed to Dalton. There is another, sharper stoichiometric law, which states that elements come together to form compounds in the ratio of their equivalent weights, and which originated with Jeremias Benjamin Richter (1792). The law of constant proportions was intensively reviewed twice more in the 19th century, by Jöns Jakob Berzelius (from 1810) and Jean Servais Stas (1860, 1865), who carried out very precise atomic weight determinations.
These observations made by Proust and others were an essential step in the development of the atomic hypothesis by Dalton. For the mass ratio of the elements in sodium chloride, which is always the same, can be explained most simply with it: Sodium chloride is composed of equal numbers of sodium and chlorine particles. A chlorine particle is half as heavy as a sodium particle. Other examples would be copper(I) sulfide (mass ratio m(Cu) : m(S) about 4 : 1, mass ratio n(Cu) : n(S) = 2 : 1; a copper particle is twice as massive as a sulfur particle) or sulfuric acid (with n(H) : n(S) : n(O) = 2 : 1 : 4, cf. figure and - on the difference between mass and mass - under list of orders of magnitude of mass).
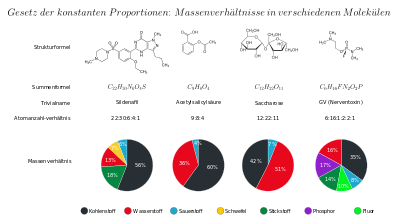
Illustration of the law of constant proportions using the mass ratios of various molecules.
See also
- Law of conservation of mass
Search within the encyclopedia