Antibody
![]()
This article is about antibodies from a biological point of view. For the feature film of the same name, see Antibodies (film).
Antibodies (immunoglobulins, in international usage also immunoglobulin, outdated gamma globulin) are proteins (proteins) from the class of globulins, which are formed (synthesized) in vertebrates as a reaction product of special body cells (plasma cells) to certain substances (called antigens). Antibodies serve the immune system. Antibodies are produced by a class of white blood cells, the plasma cells, in response to a reaction of the B lymphocytes.
Almost exclusively macromolecules or molecules bound to particles, for example lipopolysaccharides on the surface of bacteria, act as antigens. A specific antigen usually induces the formation of only a few, very specific, matching antibodies, which usually only recognize this foreign substance via specific, non-covalent binding (the fact that related targets can also be recognized has been exploited, for example, in smallpox vaccination: the antibodies formed by the body against harmless cowpox also recognize smallpox viruses that are pathogenic for humans). The specific binding of antibodies to antigens forms an essential part of the defence against invading foreign substances. In the case of pathogens as foreign substances, the formation and binding of antibodies can lead to immunity. Antibodies are central components of the immune system of higher vertebrates.
Antibodies, as first described in 1948 by the Swedish immunologist Astrid Fagraeus, are secreted by a class of white blood cells (leukocytes) called effector cells or plasma cells, which are differentiated B lymphocytes. They are found in the blood and in the extracellular fluid of the tissues and usually do not "recognize" the entire structure of the antigen, but only a part of it, the so-called antigenic determinant (the epitope). The specific antigen binding site of the antibody is called the paratope. On contact with the antigen, the antibodies generate the so-called humoral immune response (humoral defence).

IgG sculpture Angel of the West by Julian Voss-Andreae in front of the Scripps Research Institute in Jupiter, Florida. The sculpture places the main chain (using the structure published by E. Padlan) in a ring instead of a human like Leonardo da Vinci's Vitruvian Man to show the similarity between antibodies and the human body.
Structure of antibodies
Since some amino acid residues carry sugar chains, antibodies belong to the group of glycoproteins. Each antibody consists of two identical heavy chains (H) and two identical light chains (L), which are linked to each other by covalent disulfide bridges between the chains (so-called interchain disulfides) to form a ypsilon-shaped structure. The light chains (also: light chains) each consist of one variable and one constant domain. They are referred to as VL and CL. The heavy chains (also known as heavy chains), on the other hand, each have one variable and three (IgG, IgA) or four (IgM, IgE) constant domains. These are designated analogously as VH and CH1, CH2, CH3.
The variable domains of a light and a heavy chain together form the antigen binding site. The constant domain CH2 also consists of, among other things, a carbohydrate chain that forms a binding site for the complement system. The constant domain CH3 is the Fc receptor binding site for opsonization. The variable domains in turn form various characteristic paratopes, which together form an idiotype.
The two light chains are either of type κ or λ, depending on the organism and immunoglobulin subclass, and together with the portion of the heavy chains above the hinge region form the antigen-binding fragment Fab, which can be enzymatically cleaved from the underlying crystallisable fragment Fc with the aid of papain. The organism achieves the exceptional variability of the antigen binding sites (complementarity determining region, CDR) by means of V(D)J recombination.
Papain cleaves above the interchain disulfide bridges of the two heavy chains to each other. Thus, two Fab fragments and one complete fragment Fc are obtained. Pepsin, on the other hand, cleaves below the disulfide bridges. The hinge region remains between the two Fab fragments. This fragment is then called F(ab)2. Pepsin and plasmin also cleave the Fc fragment between the second and third domains of the constant part of the heavy chain.
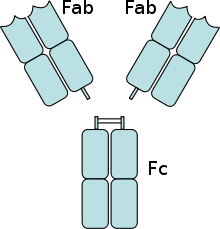
Antibody digested with papain (two 50-kDa Fab fragments and one 50-kDa Fc fragment)
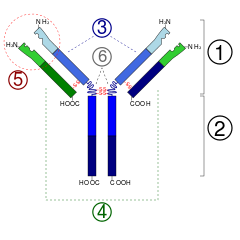
Structure of a typical IgG antibody1 . Fab section2 . Fc section3 . Heavy chains4 . Light chains5 . Antigen binding site (paratope) 6. hinge region (*) -S-S-disulfide bridge
Antigen - antibody - binding
Antibodies bind "their" paratope relatively specifically with their A(ntigen)B(inding) region, analogous to the lock-and-key principle. However, it is not uncommon that, metaphorically speaking, a second or third key exists that fits into the antibody "lock" due to the (coincidentally) similar or identical configuration of the epitope. With very low probability, this may also be an endogenous structure. One approach to explaining autoimmune diseases is based on this phenomenon.
The binding between epitope and immunoglobulin is non-covalent and subject to the law of mass action. Effective agglutination, i.e. agglutination through the formation of large complexes, is therefore only possible with approximately the same number of epitopes and binding sites. In the case of large deviations upwards or downwards, the complexes remain in solution; nevertheless, neutralisation of the effect of the antigens usually occurs. Neutralizing antibodies effective against several virus strains are called broadly neutralizing antibodies, e.g. broadly neutralizing anti-HIV antibodies.
Questions and Answers
Q: What are antibodies?
A: Antibodies are large Y-shaped proteins that can stick to the surface of bacteria and viruses. They are found in the blood or other body fluids of vertebrates, and they play a key role in the adaptive immune system.
Q: How do antibodies work?
A: Each tip of the "Y" of an antibody contains a structure (like a lock) that fits one particular key-like structure on an antigen. This binds the two structures together, allowing them to tag microbes or infected cells for attack by other parts of the immune system, or to directly neutralize their target.
Q: What is humoral immunity?
A: Humoral immunity is when antibodies are produced in response to foreign antigens entering the body. It is part of the adaptive immune system which helps protect against disease and infection.
Q: Are all antibodies different?
A: Yes, each antibody is designed to attack only one kind of antigen (in practice, this means virus or bacteria). For instance, an antibody designed to destroy smallpox is unable to hit the bubonic plague or the common cold. Though they have a similar general structure, there is variation at their tips which allows millions of different variants with different tip structures to exist so that they can bind with different antigens.
Q: How does this diversity help our bodies?
A: This enormous diversity of antibodies allows our immune systems to recognize an equally wide variety of antigens so that it can better protect us from disease and infection.
Q: Where do we find antibodies?
A: Antibodies are found in the blood or other body fluids of vertebrates such as humans and animals.
Search within the encyclopedia