Hemostasis
Haemostasis (composed of Ancient Greek αἷμα haíma, German 'blood', and stasis from στάσις stasis, German 'Stauung', 'stilling', 'stagnation', 'standstill') is a vital process that brings the bleeding that occurs when blood vessels are injured to a halt. This prevents excessive leakage of blood from the circulation and sets the stage for wound healing. Hemostasis must begin sufficiently quickly in the event of an injury to prevent major blood loss. It must be limited to the area of injury and must not be falsely triggered by other events such as inflammation or infection.
Hemostasis can be divided into two sub-processes, which, however, interact with each other. In primary (also: cellular) haemostasis, the (physiological) haemostasis, the blood platelets (thrombocytes), the wall cells of the affected blood vessel (endothelium and smooth muscle cells), as well as tissue outside the vessel are involved. In simplified terms, the vessel first narrows, then platelets attach to the leak, sticking to each other and creating the initial wound closure. In secondary (also: plasmatic) haemostasis, the clotting of blood, this still loose closure is reinforced by the formation of fibrin threads. The activation of about a dozen clotting factors contained in the blood plasma plays an important role in this process. A genetic defect in coagulation factors can lead to diseases such as haemophilia. The onset of wound healing is initiated by growth factors released by platelets and endothelial cells. At the end of wound healing, the fibrin is dissolved by the fibrinolytic system of the blood plasma.
Hemostasis is not infrequently referred to as hemostasis with its two parts, primary and secondary hemostasis.
Hypercoagulability is the increased coagulability of the blood. Haemostaseology is the study of haemostasis. Coagulopathy or a blood coagulation disorder is understood to be a pathologically altered hemostasis.
This article describes hemostasis in humans. The statements apply predominantly to other mammals, but only to a limited extent to other classes of animals.
Physiological processes after vascular injury
After injury to smaller vessels, bleeding usually stops quickly. The hemostasis responsible for this can be considered as a sequence of the following processes. This subdivision is primarily for ease of understanding. There are close functional and temporal relationships between the processes, a sharp demarcation is not possible.
Spontaneous arterial hemostasis
Arteries of the muscular type have the property to "turn in" by themselves after a transverse transection. This property is due to the wall structure of the arteries: The elastic inner skin of the artery (membrana elastica interna) contracts more than the other wall layers after transection. As a result, the free edge of the severed artery is drawn into the interior of the vessel, thus ensuring a very rapid, provisional closure.
Cellular hemostasis
It consists of the adhesion and aggregation of platelets, the activation of further platelets and the formation of an occlusive white platelet thrombus. In addition, the release of substances triggers vasoconstriction, i.e. vasoconstriction. This reduces blood flow and thus minimizes blood loss.
Plasmatic hemostasis
Components of the blood plasma create a meshwork of mechanically stable fibrin threads, in which the circulating red blood cells (erythrocytes) get caught and eventually a red thrombus forms, which finally solidifies and contracts.
Cellular hemostasis
The blood of a human normally contains between 150,000 and 400,000 platelets per microliter. The cell membrane of platelets contains numerous glycoproteins and receptors that play an important role in cellular hemostasis.
The inner cell layer of blood vessels is called endothelium. This is covered on the inside with a glycocalyx, a kind of mucus layer for which platelets have no receptors. For this reason, among others, platelets remain inactive in uninjured vessels and cannot attach to the vessel wall. Various factors also counteract activation, for example prostacyclin and nitric oxide as well as heparin, which is produced by mast cells, among others, and whose inhibitory effect on hemostasis can be used therapeutically.
Platelet adhesion and activation
When a vessel is injured, the blood comes into contact with the surrounding connective tissue, including collagen fibers. Collagen is a structural protein that is present almost everywhere in the extracellular space. Platelets first adhere to these fibers (platelet adhesion), resulting in the formation of a thin covering of the wound. Adhesion is mediated by Von Willebrand factor (vWF), a soluble blood protein produced by endothelial cells and megakaryocytes. It, together with fibronectin and laminin, provides a link between collagen fibers and a receptor on platelets (GP Ib/V/IX). A defect of the Von Willebrand factor leads to Willebrand-Jürgens syndrome.
Platelet activation is triggered by adhesion: they release calcium ions, ADP, serotonin, thromboxane A2 and other substances from so-called "electron-dense granules". This attracts further platelets (chemotaxis). Thromboxane A2 also contributes significantly to the constriction of the blood vessel, which counteracts high blood flow. The contents of the so-called "α-granules" of platelets are also secreted: clotting factors (factor V, factor VIII), adhesives (vWF, fibronectin, thrombospondin) and growth factors. Activation of various metabolic pathways leads to increased production of substances such as thromboxane A2 and platelet activating factor (PAF). Some of these substances induce plasmatic coagulation.
Platelet aggregation
→ Main article: Platelet aggregation
The aggregation of activated platelets is promoted by a reorganization of the cytoskeleton, which causes an increase in cell surface area several times over. While inactive platelets have a lenticular shape, in the active state they assume a spherical shape, bearing long pseudopodia (mock feet) that enable them to hook onto each other - the platelets become "spiky" and "sticky". The aggregated platelets eventually form a platelet plug called a white thrombus. This marks the end of cellular hemostasis. Normally the process takes one to four minutes, this duration is called the bleeding time.
The white thrombus is not too stable and can be washed away. A tighter seal is formed by plasmatic hemostasis.
Plasmatic hemostasis
Plasmatic haemostasis results in the formation of a meshwork of mechanically stable fibrin into which red blood cells (erythrocytes) are trapped in addition to platelets, hence the term "red thrombus".
Activated platelets have a receptor complex (glycoprotein IIb/IIIa) on the cell membrane to which fibrinogen from the plasma and the adhesives released from the activated platelets (fibronectin, thrombospondin) bind. Feedback mechanisms of the released substances finally lead to an irreversible aggregation in which the cell membranes of the platelets fuse together.
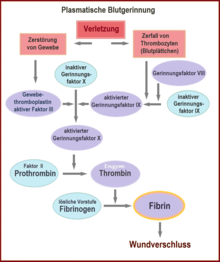
This secondary hemostasis, blood clotting, is also called the coagulation cascade. It is divided into three phases: Activation, coagulation and retraction phase.
Activation phase
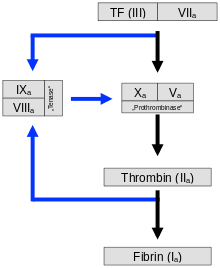
The contact of platelets with negatively charged surfaces such as glass activates factors XII and XI, which initiate a coagulation cascade (intrinsic system, see figure). If factor XII is not produced in an individual, this does not result in any significant disturbance of coagulation, in contrast to the deficiency of factors VIII, IX and XI, which leads to haemophilia A, B and C, respectively.
The normal physiological process (extrinsic system or exogenous mechanism) is initiated by contact of blood with tissue thromboplastin from injured subendothelial tissue. Tissue factor (also tissue factor (TF), tissue thromboplastin or factor III) is a membrane protein that occurs, for example, in the adventitia of blood vessels - it is released by endothelial cells only after activation. It forms a complex with factor VII, which is converted into its active form. This produces some thrombin, but the process is inhibited relatively quickly by TFPI (tissue factor pathway inhibitor). When enough thrombin has been produced, a so-called activator complex of factors IX and VIII is activated (see IXa and VIIIa in figure). This complex in turn activates factor X.
The absence of factors VIII or IX leads to haemophilia, the bleeding disorder: the cascade is interrupted and the amplification of clotting fails to occur. Patients can bleed to death from the smallest internal injuries.
In both mechanisms - intrinsic and extrinsic pathway - factor X is finally activated to factor Xa. This in turn cleaves prothrombin (factor II), resulting in thrombin (factor IIa). This reaction on the platelet membrane takes place only in the presence of calcium and is strongly accelerated by positive feedback with the complex of factors VIII and IX. The activation phase ends with the formation of enzymatically active thrombin.
Phases of coagulation and retraction
The enzymatically active thrombin is responsible for the polymerization of fibrin and thus the formation of the red thrombus: In the coagulation phase, it splits off low-molecular units (monomers) from the inactive precursor fibrinogen (factor I), which assemble non-covalently to form the polymeric fibrin. Through the action of factor XIII, covalent bonds are finally formed between the monomers and the thrombus is stabilized. The fibrin cross-links the platelets that are already attached to each other and thus strengthens the wound closure. Red blood cells are caught in the network, a so-called red thrombus is formed. The thrombin also causes a contraction of the actin-myosin skeleton within the platelets: the contracting platelets pull on the fibrin net and thus the wound edges together and mechanically close the wound. The contraction - supported by PDGF (platelet-derived growth factor) - also promotes the penetration of connective tissue cells: wound healing begins.
New cell-based model of coagulation
The classical model of coagulation distinguishes between intrinsic and extrinsic activation and thus describes a multistep sequence of protein activation in cell-free plasma. The classical coagulation tests aPTT and PT correspond to this conception. This model is not suitable for describing blood coagulation at damaged blood vessels in the body, so that a cell-based model of coagulation was established in 2001, which describes three overlapping phases:
Initiation
Due to a tissue injury, cells that are otherwise located outside the blood vessel come into contact with the blood stream below the vascular endothelium. The cells now lying open in the bloodstream carry tissue factor (III) on their surface (tissue-factor-(III)-bearing cell). The complex of tissue factor (III) and proconvertin (VII) now catalyzes the activation of thrombokinase (X), which for the time being can only activate small amounts of thrombin (II). However, this small amount of thrombin (II) is sufficient to activate platelets as well as the coagulation factors proaccelerin (V) and proconvertin(VII), thus triggering the amplification of thrombin formation. This first phase takes place in a small space at the subendothelial injury.
Amplification
The proaccelerin (V) activated in the initiation phase forms the prothrombinase complex (X,V) with thrombokinase (X), which can activate thrombin (II). Overlapping in time is the amplification phase, in which platelets attach themselves to subendothelial structures (adhesion). This occurs via GPVI receptors that bind to collagen and via the GPIb/IX receptor that binds to Von Willebrand factor. In this process, the Von Willebrand complex releases the bound factor VIII, which binds in active form to the surface of the platelets. Furthermore, the platelet releases its internal stores, which include proaccelerin (V). Tissue factor(III) and proconvertin(VII) activate not only thrombokinase (X) but also serine protease (IX). The first small amounts of thrombin (II) activate not only fibrin (I) but also factor (VIII). The tenase complex formed from VIII and IX in turn activates thrombokinase (X), forming a self-reinforcing loop. This important step is also known as the Josso loop. Now, on the surface of the subendothelial injury as well as on the surface of the platelet adhering to it, numerous clotting factors are present in high concentrations and are protected from the anticoagulant proteins in the free blood.
Propagation
The factors accumulated on the platelet surface form tenase complexes (VIII, IX), which support the formation of the prothrombinase complex (X, V). Large amounts of thrombin (II) are now activated by thrombokinase (X) (thrombin burst). Thrombin (II) finally forms the fibrin networks into which platelets bind with their GPIIb/IIIa receptors. Factor XIII stabilizes these networks by additional fibrin cross-links.
Control against unwanted spread of the clot
To prevent a clot from forming outside the endothelial injury (thrombosis), the endothelium and the free-flowing blood have several mechanisms: Dissolving thrombin (II) is rapidly deactivated in the bloodstream by the anticoagulant protein antithrombin. Dissolving thrombokinase (X) and proconvertin (VII) is bound by TFPI. On the surface of the endothelium, thrombomodulin (TM) binds trombin (II) so that it can no longer form fibrin (I). At the same time, the formation of protein C (APC) is increased a thousandfold by the bound thrombin (II), so that thrombin now has an anticoagulant effect. Protein C (APC) then forms a complex with protein S that deactivates coagulation factors V and VIII. Furthermore, the endothelium has membrane-bound ADPases that degrade ADP and thus downregulate platelet function.
Transition to wound healing
→ Main article: Wound healing and fibrinolysis
After plasmatic hemostasis, wound healing takes place as connective tissue-forming cells (fibroblasts) grow into the thrombus and remodel it into connective tissue. In the process, damaged cells die and are degraded. A protein called plasmin is primarily responsible for the degradation of thrombi. Plasmin is also formed from an inactive precursor (plasminogen) by complicated regulated mechanisms. Plasmin breaks the covalent bonds between the fibrin strands and thus the network that holds the thrombus in place.
There are coordinated equilibria between the systems of blood clotting and fibrinolysis, which dissolves the red thrombus in the vascular system. Minor disturbances of these equilibria can lead to serious bleeding or to the formation of thrombi in places where there is no injury (see also thrombosis).
Tissue factor (TF, III) and proconvertin (VII) activate not only thrombokinase (X) but also serine protease (IX). The first small amounts of thrombin (II) activate not only fibrin (I) but also factor (VIII). The tenase complex formed from VIII and IX in turn activates thrombokinase (X), forming a self-reinforcing loop. This important step is also known as the Josso loop.
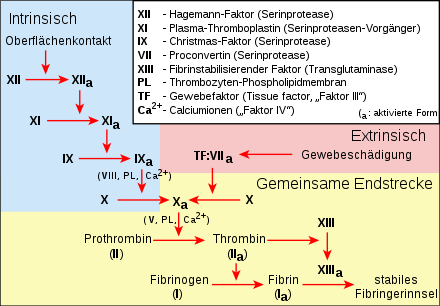
Extrinsic and intrinsic coagulation system with the common end pathway in a different representation
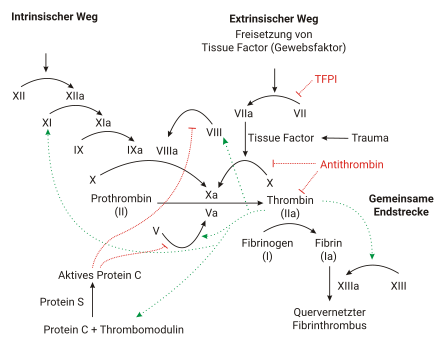
The coagulation cascade
Running blood on a fresh cut

Platelet-rich blood plasma (left) is a turbid liquid. When ADP is added, the platelets are activated and bind to each other, forming white flocs (right).
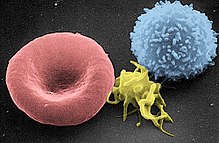
From left to right: erythrocyte, activated platelet, leukocyte.
A separate gene is responsible for the formation of each of the enzymes (clotting factors). Only the expression of all these genes enables the effects of all these enzymes, which gradually lead to blood clotting. The end product, fibrin, accomplishes a natural wound closure. (See also gene chain of action and polygeny).
Overview of coagulation factors and inhibitors
Except for calcium ions (factor IV), the clotting factors are proteins. Each factor is assigned a Roman numeral. A small a after the number means that it is in the active form. For historical reasons (see under History of Research), the number VI is not (no longer) assigned, the corresponding factor is identical to Va.
| Number | Name(s) | Functions | Deficiency Syndromes |
| I | Fibrinogen | Precursor molecule for the formation of the fibrin network. | Afibrinogenemia (congenital or in consumptive coagulopathy) |
| II | Prothrombin | The active form thrombin (IIa) activates factors I, V, VIII, XI and XIII. | Hypoprothrombinemia (congenital, vitamin K deficiency or in consumptive coagulopathy) |
| III | Tissue factor, tissue thromboplastin, tissue factor (TF) | The only one not in the blood, but in the subendothelial tissue. TF and VIIa form with Ca2+ the extrinsic tenase that activates X. | |
| IV | Calcium | Many factors require the calcium cation Ca2+ to bind to the negatively charged phospholipids of plasma membranes. | Calcium deficiency |
| V | Proaccelerin | Va and Xa form with Ca2+ and phospholipids the prothrombinase complex that activates II. | Parahemophilia (congenital) |
| VI | corresponds to factor Va | ||
| VII | Proconvertin | VIIa and TF form with Ca2+ the extrinsic tenase that activates X. | Hypoproconvertinemia (congenital, vitamin K deficiency) |
| VIII | Antihemophilic globulin A | VIIIa and IXa form with Ca2+ and phospholipids the intrinsic tenase that activates X. | Haemophilia A (congenital, X-linked recessive inheritance) |
| IX | Christmas Factor, Antihemophilic Globulin B | VIIIa and IXa form with Ca2+ and phospholipids the intrinsic tenase that activates X. | Haemophilia B (congenital, X-linked recessive inheritance) |
| X | Stuart Prower Factor | Va and Xa form with Ca2+ and phospholipids the prothrombinase complex that activates II. | Factor X deficiency (congenital) |
| XI | Rosenthal Factor, Plasma Thromboplasmin Antecedent (PTA) | XIa activated IX. | Haemophilia C (congenital) or PTA deficiency in consumptive coagulopathy |
| XII | Hageman factor | XIIa activated XI. | Hageman's syndrome is more likely to lead to disturbances in fibrinolysis (congenital or in consumptive coagulopathy) |
| XIII | fibrin stabilizing factor | XIIIa converts fibrin monomers into cross-linked fibrin. | Factor XIII deficiency |
To prevent clotting in the absence of injury, blood plasma contains various inhibitory substances (inhibitors). Protease inhibitors inhibit the formation of fibrin. Antithrombin inhibits several coagulation proteases in the activation phase and coagulation phase. The inhibitory effect is significantly enhanced by its cofactor, heparin. Heparin is produced by endothelial cells and mast cells. Thrombomodulin, which also originates from the endothelium, binds to thrombin and activates protein C, which, after binding to protein S, inactivates cofactors Va and VIIIa.
Search within the encyclopedia