Helium
![]()
This article describes the chemical element. For the short film of this name, see Helium (short film).
Helium (ancient Greek ἥλιος hélios, German 'Sonne') is a chemical element and has atomic number 2. Its element symbol is He. In the periodic table, it is in the 18th IUPAC group, formerly VIII. Main Group, and thus belongs to the noble gases. It is a colourless, odourless, tasteless and non-toxic gas.
Helium remains gaseous down to very low temperatures, only becoming liquid near absolute zero. It is the only substance that does not become solid even at absolute zero (0 K or -273.15 °C) under normal pressure. Besides neon, helium is the only element for which, even under extreme conditions, no compounds have yet been detected that did not decay immediately after formation. Helium occurs only atomically. The most common stable isotope is 4He; another stable isotope is 3He, which is extremely rare on Earth.
The behaviour of the two liquid phases helium I and helium II (rsp. helium-I and helium-II) (in particular the phenomenon of superfluidity) of 4He is the subject of current research in the field of quantum mechanics. Liquid helium is an indispensable tool for achieving very low temperatures. These are required, among other things, for cooling infrared detectors of space telescopes and for studying properties such as the superconductivity of matter at temperatures close to absolute zero.
Helium is the second most abundant element in the universe after hydrogen and accounts for about a quarter of the total mass of matter in the universe. According to accepted theory, protons and neutrons combined to form the first atomic nuclei through nuclear fusion about ten seconds after the Big Bang. About 25% of their total mass is 4He, 0.001% deuterium, and traces of 3He. Thus, most of the helium was already formed in the Big Bang. Helium, which was later formed inside stars by fusion of hydrogen, continued to fuse to heavier elements for the most part.
On Earth, 4He is formed in the form of alpha particles during the alpha decay of various radioactive elements such as uranium or radium. Helium is formed when the alpha particle snatches two electrons from other atoms. Most of the helium present on Earth is therefore of non-stellar origin. The helium thus formed accumulates in natural gas deposits in concentrations of up to 16 percent by volume. Therefore, helium can be extracted from natural gas by fractional distillation.
The first evidence of helium was discovered in 1868 by the French astronomer Jules Janssen during investigations of the light spectrum of the chromosphere of the Sun, where he found the hitherto unknown yellow spectral line of helium.
Helium is used in cryogenic technology, especially as a coolant for superconducting magnets, in deep-sea breathing apparatus, in the determination of the age of rocks, as a filling gas for balloons, as a lifting gas for airships and as a protective gas for various industrial applications (for example, in metal inert gas welding, as a carrier gas in capillary gas chromatography and in the production of silicon wafers). After inhaling helium, the voice changes briefly ("Mickey Mouse voice") due to the higher speed of sound compared to air.
History
Evidence of the element helium was first obtained due to a bright yellow spectral line at a wavelength of 587.49 nanometers in the spectrum of the chromosphere of the Sun. This observation was made by the French astronomer Jules Janssen in India during the total solar eclipse of August 18, 1868. When he made his discovery known, no one believed him at first, since no new element had ever been found in space before evidence was found on Earth. On October 20 of the same year, Norman Lockyer, an Englishman, confirmed that the yellow line was indeed present in the solar spectrum, and concluded that it was caused by a previously unknown element. Since this spectral line was very close (1.8 nm from the center) to the Fraunhofer double-D line (D2 = 589.00 nm, D1 = 589.60 nm) of the metal sodium, he named the line D3 to distinguish it from these lines D1 and D2 of sodium. He and his English colleague Edward Frankland proposed to call the new element helium (from Greek helios, sun).
14 years later, in 1882, Luigi Palmieri succeeded for the first time in detecting the element helium on Earth by spectral analysis of Vesuvius lava.
On March 23, 1895, British chemist William Ramsay obtained helium by adding mineral acids to the uranium mineral cleveite, a variety of uraninite, and isolating the resulting gas. He was looking for argon, but was able to observe the yellow D3 line after separating nitrogen and oxygen from the isolated gas. The same discovery was made almost simultaneously by the British physicist William Crookes and the Swedish chemists Per Teodor Cleve and Nicolas Langlet at Uppsala in Sweden. They collected sufficient quantities of the gas to be able to determine its atomic mass.
During oil drilling at Dexter in Kansas, a natural gas well was found to contain 12 percent by volume of an unknown gas. In 1905, American chemists Hamilton Cady and David McFarland of the University of Kansas discovered that it was helium. They published a report that helium could be extracted from natural gas. In the same year, Ernest Rutherford and Thomas Royds determined that alpha particles are helium nuclei.
The first liquefaction of helium was carried out in 1908 by the Dutch physicist Heike Kamerlingh Onnes, by cooling the gas to a temperature of less than 1 K. He was also unable to obtain solid helium by further cooling. He was unable to obtain solid helium even with further cooling; this was only achieved in 1926 by Willem Hendrik Keesom, a student of Kamerlingh Onnes, by compressing the helium to 25 bar at an analogous temperature. Kamerlingh Onnes first described the phenomenon of superfluid liquids, known today as the Onnes effect.
In the early 20th century, large quantities of helium were found in natural gas fields of the American Great Plains, making the United States the leading world supplier of helium. Following a proposal by Sir Richard Threlfall, the U.S. Navy sponsored three small experimental helium production operations during World War I to produce helium as a filler gas for barrage balloons. A total volume of 5,700 cubic meters of gas containing 92% helium was recovered from these operations. This helium was used in the first helium-filled airship, the US Navy's C-7, in 1921.
The U.S. government established the National Helium Reserve at Amarillo, Texas, in 1925 to provide a supply for military airships in wartime and commercial airships in peacetime. The storage facility is located in a natural rock formation 20 km northwest of Amarillo. Although demand declined after World War II, the Amarillo production facility was expanded to provide liquid helium as a coolant for oxygen-hydrogen rocket fuel and other items requiring cooling. U.S. helium consumption in 1965 increased to eight times peak wartime consumption.
After the Helium Acts Amendments of 1960 (Public Law 86-777) were passed in the USA, another five private helium production facilities were built. For this purpose, the US Department of Mines had a 685-kilometer pipeline built from Bushton in Kansas to Amarillo in Texas; this storage facility contained about one billion cubic meters of helium in 1995 and about ten times the world's annual helium demand in 2004. By 2015, the storage facility is to be empty and dissolved (Helium Privatization Act).
The purity of the helium obtained increased rapidly after the Second World War. Whereas in 1945 a mixture of 98 % helium and 2 % nitrogen was still used for airships, by 1949 helium with a purity of 99.995 % could already be sold commercially. To achieve this degree of purity, activated carbon is required to remove remaining impurities - mostly consisting of neon - by means of pressure swing adsorption.

Sir William Ramsay
Jules Janssen, first discoverer of helium
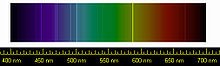
The spectral lines of helium. The yellow spectral line is particularly striking.

The complete spectrum of the sun
Spectrum of a helium gas discharge
Occurrence
Space
According to the Big Bang theory, most of the helium present in space today was formed in the first three minutes after the Big Bang. Helium is the second most abundant element after hydrogen. 23% of the mass of visible matter is made up of helium, although hydrogen atoms are eight times more abundant. Helium is also produced by nuclear fusion in stars. This process, called hydrogen burning, provides the energy that makes stars on the main sequence, the majority of all stars, glow. This process provides stars with the energy for most of their life. When most of the hydrogen is used up in the core at the end of a star's life, the core contracts and raises its temperature. This now allows helium to burn into carbon (helium flash, helium burning). Hydrogen burning continues to take place in a shell around this core. Carbon can also continue to burn to other elements. This process usually continues until iron is formed, unless a supernova explosion occurs. A supernova explosion also synthesizes heavier elements than iron, which are dispersed in space by the explosion. Over time, this enriches the interstellar matter with helium and heavier elements, so that later resulting stellar populations also have a greater proportion of helium and heavier elements.
On stellar surfaces and in nebulae, helium occurs preferentially in a neutral or singly ionized state. Unlike usual in physics and chemistry, the notation with superscript "+" (He+) is not used in astronomy, because other elements can occur so highly ionized that this notation becomes impractical (e.g. sixteenfold ionized iron in the solar corona). Ionization levels are designated in astronomy by Roman numerals, with neutral helium being designated He I, singly ionized corresponding to He II, and fully (= doubly) ionized as Helium III (Helium-III).
Helium is also present in planetary atmospheres in varying proportions. The following is an example of the material quantity fraction near the ground or, in the case of the gas planets, on the outside:
| Neptune | 19 % ± 3,2 % |
| Uranus | 15,2 % ± 3,3 % |
| Jupiter | 10,2 % |
| Saturn | 03,25 % |
| Venus | 00.0012 % (12 ppm) |
| Earth | 00.00052 % (5.2 ppm) |
Meteorites, asteroids and moon
Helium can be produced in meteorites and surface lunar rocks also by interaction (spallation) with cosmic rays. Especially 3He can therefore be used to determine the so-called irradiation age, which usually corresponds to the period from the meteorite's breakaway from the parent body until its arrival on Earth. Besides 4He is formed in meteorites by decay of heavy radioactive elements. Also there are further helium parts in meteorites, which originate from the time of the formation of the solar system.
The bulk of the helium also bound in the moon's regolith comes from the solar wind when it hits the surface unimpeded by an atmosphere or magnetic field. About 4 % of the solar wind is helium ions, of which about 0.48 ‰ is helium-3. The helium ions of the solar wind have an energy of about 3 keV, penetrate solids and remain there (→ ion implantation). Helium is found especially in the fine fraction of regolith at the surface because of the shallow ion penetration depth (sub-micrometer range) and because of mixing to depths of several meters. It is particularly abundant in titanium oxide-rich conductive minerals (ilmenite). It occurs here in concentrations up to 70 mass ppm. About 100 ppm of the helium bound in the moon rock is the isotope helium-3, which is extremely rare on Earth and whose use in fusion reactors is under discussion.
Earth
4He is formed in the Earth's body during the radioactive decay (alpha decay) of heavy elements such as uranium or thorium, where helium nuclei are emitted as alpha particles and subsequently capture electrons. It can be found in various uranium- and thorium-bearing minerals such as pitchblende.
From the time of the Earth's formation comes a fraction of 3He in the mantle that is far above the atmospheric value, the so-called mantle helium; the 4He/3He ratio in the upper mantle, which is largely degassed and whose helium stock is therefore essentially replenished by 4He from alpha decays, is about 86. 000.000. If the convection system of the lower mantle is largely separated from that of the upper and the mass exchange between the two is correspondingly low, the ratio in the lower, barely degassed mantle is between 2500 and 26,000, i.e., the fraction of 3He is higher. This is of particular geodynamic interest with respect to the causes of hotspot volcanism: while 4He/3He = 86,000 is typical for basalts from mid-ocean ridges formed by melting processes of upper mantle material, basalts from some hotspots, for example oceanic volcanic islands such as Hawaii and Iceland, are about three to four times richer in 3He. This is commonly explained by the fact that this volcanism is caused by mantle plumes originating at the core-mantle boundary and therefore consisting at least in part of lower mantle material.
Helium occurs - by the same mechanism of accumulation - in natural gas (with up to 16% by volume) and in small quantities in crude oil (0.4%). European natural gas deposits contain only 0.12 (North Sea) to 0.4 percent by volume (Poland), while up to 16 percent by volume is possible in Siberian, North American (Canada, Texas, Kansas and Oklahoma) and Algerian natural gas deposits.
In lower layers of the Earth's atmosphere, especially the troposphere which is mixed by the weather, the helium content is about 5.2 ppm. At very high altitudes, gases tend to segregate according to their different densities, even against the mixing effect of the undirected molecular heat movement. Above 100 km altitude (homosphere), the atmosphere is increasingly segregated, and helium becomes the predominant gas (in terms of number of particles) at altitudes >400 km. At these altitudes, helium atoms escape into space - in the stationary case as much as is supplied from the Earth's surface by diffusion, transport and volcanism.

The earth produces helium through radioactive processes in the earth's interior.

Helium makes up about 19% of Neptune's outer gas layers. Neptune's main constituent is hydrogen; the blue-green coloration is due to methane.
Questions and Answers
Q: What is the chemical symbol for helium?
A: The chemical symbol for helium is He.
Q: How many isotopes of helium are there?
A: There are 9 isotopes of helium, with only two being stable.
Q: What makes helium a noble gas?
A: Helium is called a noble gas because it does not regularly mix with other chemicals and form new compounds.
Q: What color and smell does helium have?
A: Helium has no color or smell. However, when placed in an electric field, it has a red-orange glow.
Q: When was the presence of helium first detected by astronomers?
A: Astronomers first detected the presence of helium in 1868 when its spectrum was identified in light from the Sun. This was before its discovery on Earth.
Q: What are some uses for helium on Earth?
A: On Earth, helium is used to fill balloons and airships due to its lighter density than air, as well as in some kinds of light bulbs. It can also be breathed in but this can be dangerous if too much is inhaled as it can cause hypoxia which can injure or kill someone who isn't breathing normal air and long-term effects to vocal cords.
Q: How is helium created naturally on Earth?
A: On Earth, helium is made by the natural radioactive decay of heavy radioactive elements like thorium and uranium, although there are other examples. The alpha particles emitted by such decays consist of helium-4 nuclei.
Search within the encyclopedia