Amyloidosis
![]()
This article describes the clinical picture of the same name. The stainability of organic samples with iodine reagents is dealt with under amyloidity.
Amyloidosis (from the ancient Greek ἄμυλον ámylon "power meal, starch") is a variable disease with accumulation of (sometimes abnormally altered) proteins mostly extracellularly in the interstitium (intercellular space). These insoluble deposits are in the form of small fibers called fibrils (β-fibrils) and are known as amyloid. Amyloid is detected by microscopic examination of tissue samples previously stained with Congo red. The amyloid deposits appear bright red in bright field, in polarized light they show an apple-green birefringence.
The deposition of corpora amylacea in the prostate is considered the only non-pathological form of amyloid deposition in the body. Amyloidosis itself is not a single disease, but a pathological deposition process (due to the aforementioned fribrillar protein deposits), which is triggered by different metabolic defects and - depending on the organ affected - can lead to various chronic diseases.
Amyloid means something like "starch-like" because the deposits often show a blue coloration when iodine is added, similar to the iodine-starch reaction. Rudolf Virchow first used the term in 1854 when he found atypical material in the livers of deceased people.
The incidence of systemic amyloidosis (amyloidosis affecting the whole body as a multisystem disease) is about 1 case per 100,000 inhabitants per year. Affected are predominantly older patients around 65 years of age. The systemic disease is often fatal even with intensive treatment (cytostatics, glucocorticoids); only sometimes - depending on the biochemical cause - is it possible to treat the cause.
Today, the main forms of amyloidosis are light chain amyloidosis (AL) and transthyretin-related ATTR amyloidosis.
In untreated AA amyloidosis (a form of amyloidosis in which the insoluble fibrils are composed of amyloid A, the precursor protein of which is an acute phase protein), the median survival time is 3 to 4 years. Cures are possible if the cause was infection. Among all systemic forms of amyloidosis, AL amyloidosis is the most common.
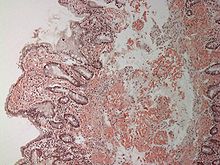
Amyloid in the connective tissue layer (lamina propria) of the duodenum. Staining with Congo red. The amyloid deposits appear as bright red, structureless material in the connective tissue and around the blood vessels.
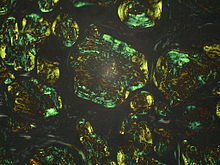
Amyloidosis - Lymph nodes in polarized light
Cause and origin
Amyloidosis is caused by a disorder in the folding of a normally soluble protein. Several different diseases can trigger the disease through overproduction, lack of/reduced degradation or impaired excretion of certain proteins. These proteins are usually present in the blood plasma in a dissolved form. However, if their concentration increases, they also reach the surrounding tissue, where enzymes attack them. The aggregation of the resulting amino acid chains in the area of the β-sheet structures results in the formation of insoluble complexes in the form of microscopic fibres (fibrils).
Since the fibrils are resistant to the defense mechanisms (phagocytosis and proteolysis), they can no longer be removed. According to recent findings, however, amyloid deposits can at least be reduced by removing the precursor proteins.
The amyloid deposits destroy the architecture of the organs and thus lead to functional disorders. There is evidence that the deposits also exert a direct toxic effect on cells.
Diagnosis
The symptoms of amyloidosis are often non-specific, especially in the early stages of the disease. For this reason, the diagnosis is often only made in an advanced stage of the disease. Amyloidosis should be considered in the presence of otherwise unexplained proteinuria, as well as in patients with cardiomyopathy, neuropathy, liver enlargement, or multiple myeloma.
Since, if scintigraphic evidence of cardiac ATTR amyloidosis (transthyretin amyloidosis) has not been obtained when a monoclonal gammopathy has been excluded, the exact composition of the amyloid must be known for treatment, a biopsy (tissue sample) for histological amyloid detection with subtyping is usually required. This can be obtained from an affected organ (kidney, heart, stomach). A recent discovery is that generalized amyloidoses can also be classified with biopsies from subcutaneous adipose tissue, a less stressful procedure for the patient. Other less burdensome sampling sites include the minor salivary glands, gums, rectum, or skin. The histologically prepared specimens are stained with Congo red and examined by immunohistochemistry. Amyloid binds the dye Congo red and then becomes visible with a greenish glow under polarized light. The electron microscope shows that the amyloid deposits consist of fibrils, irregularly arranged, thread-like structures of different lengths with a diameter between 8 and 15 nm.
If amyloidosis has been confirmed by tissue sampling, a search for light chain disease should be performed by bone marrow aspiration, blood and urine tests. In the bone marrow, a monoclonal proliferation of plasma cells, i.e. plasma cells that produce either only kappa or only lambda chains, is indicative of AL amyloidosis. The light chains can also be detected by immunoelectrophoresis in serum or urine.
The extent of amyloid deposition can be visualized by scintigraphy with radiolabeled substances that bind to amyloid. Binding to amyloid are technetium Tc 99m-pyrophosphate, technetium-labeled aprotinin, and 
In many cases, the diagnosis is made only after death by autopsy. An oversized tongue and characteristic coarse subcutaneous swellings point the pathologist to the correct diagnosis. Because of the bacon-like appearance of the cut surfaces of affected organs, they are also called bacon liver, bacon spleen, or bacon kidney. Just like Virchow, he can sprinkle the cut surface of any organ (so also tongue, heart) with Lugol's solution, which, with amyloid, gives a characteristic brown colour.
Search within the encyclopedia