Freezing
![]()
The title of this article is ambiguous. For other meanings, see rigor mortis, facial expression, stare, and rigidity.
![]()
This article or section is still missing important information. Help Wikipedia by researching and adding it.
In physics, solidification refers to the transition of a substance from the liquid to the solid state of aggregation. In most cases, this occurs through cooling. The reverse process of solidification is melting. Solidification takes place in the solidification interval.
With pure substances and constant pressure, solidification always occurs at a certain temperature, which is called the freezing point (solidification line). For pure substances, it corresponds exactly to the melting point. Although the substance gives off heat during solidification (solidification heat), the temperature remains constant during the transition from liquid to solid (latent heat). During solidification, crystallization occurs in many substances, during which the Brownian motion of the molecules is reduced. The molecules therefore have less energy in the solidified state than in the liquid state, which is equivalent to a loss of energy.
Water and other aqueous solutions freeze when they change into the solid state. Freezing is also colloquially referred to as the preservation of food by deep-freezing. In alloys and glasses, solidification begins at the liquidus temperature and is completed at the solidus temperature.

Play media file Video: Freezing water. Because of the great supercooling, the water freezes particularly quickly. The solidification is triggered by vibrations, here by mere touching.
General
Liquids can turn into solids because of various reasons and in various ways. Especially often solidification can be observed at constant pressure by cooling. If it is a pure substance, then the liquid begins to solidify when it reaches the solidification temperature and does not cool further until solidification is complete. The solidification temperature is almost always identical to the melting temperature. A change in pressure at constant temperature can also cause solidification. For most substances, this requires an increase in pressure, while for water and some other substances, a decrease in pressure can also lead to solidification. (See also anomaly of water). Boiling and condensation can also be caused by pressure changes, but for solidification much larger pressure changes are necessary. The relationship between temperature and pressure can be seen in phase diagrams. There you can also see that the solidification line, which separates the liquid area from the solid area in the diagram, is steeper than the melting line between liquid and gaseous.
For many mixtures (and thus also alloys) there is a temperature range, the solidification interval, in which the substance is both solid and liquid. Solidification begins at the liquidus temperature and ends at the solidus temperature. Both depend on the mixing ratio or the proportion of alloying elements and can also be taken from phase diagrams.
Solidification can also occur through chemical reactions. This is the case, for example, when a liquid at room temperature transforms into a substance with a solidification temperature higher than room temperature. The phenomenon also occurs in metallurgy: Liquid metals react with oxygen to form oxides. Liquid aluminum, for example, solidifies at 660 °C, while aluminum oxide is still solid at over 2000 °C.
During solidification, energy is released, the solidification heat. The same amount of energy is needed to melt the material again (heat of fusion).
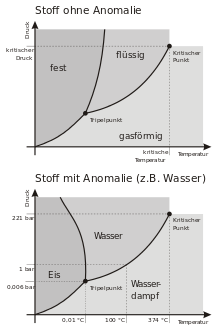
Phase diagram of an "ordinary" substance and water
Property Changes
During solidification, numerous physical properties change abruptly. Almost all physical properties change with the temperature of a body. As long as the body does not change its state of aggregation, these properties usually do not change abruptly, but continuously and very slowly. Changes in volume, density and solubility are of particular importance.
Volume and density
Most substances reduce their volume during solidification and thus increase their density (mass per volume) because of the constant mass. This phenomenon is called solidification shrinkage. Water, on the other hand, expands during solidification. This is why ice floats on liquid water, while most other substances sink in the melt. The expansion when water freezes can cause glass bottles filled with water to burst. In winter, water seeps into small cracks in pavement and rock, expanding and thus enlarging these cracks, which is called frost heave. Thermal expansion, on the other hand, is related to temperature and not to the state of aggregation.
In the foundry, the solidification shrinkage can cause damage to the castings. Especially with complex shaped workpieces, the reduction of the casting is blocked by the mould, which can lead to cracks. These are called hot cracks and can also occur during welding. In order to keep shrinkage as low as possible, silicon is often added to cast alloys, as it expands during solidification and can thus partially compensate for shrinkage.
Solubility
Solubility also changes greatly with solidification. In general, a substance can dissolve less and less of another substance as it cools. During solidification, however, the solubility drops sharply. If impurities are dissolved in a substance, they can be removed by recrystallization or the electroslag remelting process, since the impurities remain primarily in the area that has not yet solidified. In the foundry, gases are often also dissolved in the melt. When the melt cools rapidly, these gases do not have sufficient time to escape from the melt and then remain in the casting where they form bubbles and pores, which reduces the strength of the castings. When these castings are subsequently welded, the gases in the pores expand greatly and can damage the workpiece.

Aluminium casting with pores
Search within the encyclopedia