Amount of substance
The amount of substance (outdated mole amount or mole number) with the formula symbol 
The unit of measurement of the amount of substance is the mole, an SI base unit. A quantity of substance of 1 mole ( 
Substance quantity and the quantities derived from it, such as substance quantity concentration, substance quantity fraction and substance quantity ratio, are important in stoichiometry. The use of the quantity of substance shifts considerations of chemical reactions from the atomic or molecular range to weighable substance masses with a very high number of particles.
For the amount of substance nX and the mass mX of a substance portion of a substance X and its molar mass MX holds:
Calculation of the amount of substance
The calculation of the amount of substance is
... from the mass
The calculation from the mass is possible via the equation given above.
Example: The molar mass of water is 18 grams per mole. 9 grams of water thus correspond to a substance quantity of 0.5 mole.
... from the volume
At standard conditions, one mole of an ideal gas occupies a volume of Vm = 22.4 l/mol as an approximation. This volume is called the molar standard volume (molar volume). The amount of substance results directly from the volume.
Example: How many moles correspond to 5 l of oxygen?
... from the number of particles
Since the number of particles N is proportional to the amount of substance n (the proportionality factor is the Avogadro constant NA = 6.022 - 1023/mol), the amount of substance can be calculated from the number of particles.
Example: Given are N = 1025 particles.
... from concentration
Since the concentration cX (mol/l) is a concentration measure for solutions that relates the amount of substance X to the volume V of the solution, it can also be calculated back to the amount of substance.
Example: How many moles of sodium chloride are in 0.22 liters of a 0.6-molar NaCl solution?
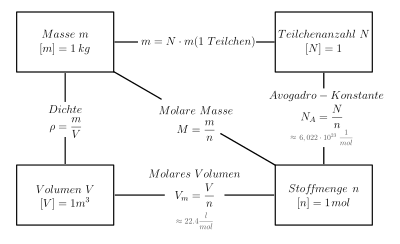
The relationship between mass, amount of substance, volume and number of particles.
See also
- Salary information
- Order of magnitude (amount of substance)
Search within the encyclopedia




