Fossil fuel
Fossil energy is derived from fuels that were created in geological prehistory from decomposition products of dead plants and animals. These include lignite, hard coal, peat, natural gas and petroleum. These energy sources are called fossil fuels or fossil fuels (see also fossil). In contrast, biomass is derived from wood and other modern organic wastes and remains.
Fossil fuels are based on the carbon cycle and thus enable stored (solar) energy of past times to be utilised today. The technical exploitation of fossil fuels, initially almost exclusively coal, has enabled continuous economic growth since the Industrial Revolution. In 2005, 81% of the world's energy needs were met from fossil sources.
The energy content of the listed fossil fuels is based on the carbon content. When burned with oxygen, energy is released in the form of heat and oxides, always including carbon dioxide. Therefore, the burning of fossil fuels is "highly polluting" both locally and globally. Fossil fuels are the main source of man-made greenhouse gas emissions and thus global warming. Depending on the composition and purity of the fossil fuel, it also produces other chemical compounds such as nitrogen oxides and soot, as well as dusts of varying fineness.
The opposite term to fossil energy is renewable energy. It is taken from energetic processes that are constantly renewed. Renewable energy includes above all the use of wind energy, solar radiation (solar collectors and solar cells) and water currents and the energy recovery of biomass, but also the use of tides and geothermal energy.
Nuclear energy is sometimes considered "fossil energy" because it is non-renewable. But uranium ore, the raw material used to make fuel rods for nuclear power plants, is not derived from decomposition products of dead plants and animals, but is an inorganic mineralization.
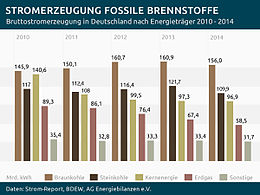
Electricity generation from fossil fuels and nuclear energy in Germany 2010-2014
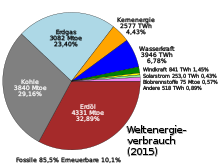
Global energy consumption by energy type 2015 (commercially traded energy only)
Supplies
Reserves and resources
The reserves of fossil fuels stored in the earth (fossil energy sources) that are proven, safely available and economically recoverable with today's technology are referred to as energy reserves. Assuming that energy demand and use remain constant, the currently known world energy reserves (as of 2020) of crude oil and natural gas will each last for about 50 years and of coal for about 130 years. However, the International Energy Agency (IEA) assumes that global primary energy demand will increase by 36 % between 2008 and 2035, but only if energy policy measures such as increasing energy efficiency and expanding renewable energies are implemented. In a comparative scenario without these measures, the increase in primary energy demand is higher. Factors affecting the availability of fossil energy sources include:
- Size of the energy reserve
- Effectiveness in the usability of energy
- Scope of consumption
- Switching to renewable resources
In addition to available energy reserves, there are proven and suspected reserves of energy carriers (so-called energy resources), which, however, are currently not yet recoverable for technical or economic reasons.
The reserves of fossil fuels will probably last for another 100 years at most. In the case of oil, the static range of US oil production, which accounted for about two-thirds of the world market, was 6 years in the mid-1920s and has since risen to about 50 years as new deposits and higher prices have been added, allowing for more expensive extraction methods.
In addition to the static range as a statistical parameter, an important factor is the point in time at which production can no longer be increased but begins to decline (production maximum). Since this changes the relationship between supply and demand, this can result in sharply rising prices. The supply gap in the area of fuels can be covered by lower consumption and alternative drives, and in the generation of electrical energy by renewable energies or nuclear energy.
Climatic effects and limits of use
| Heat release and carbon dioxide emissions from the combustion of one gram of fuel | ||
| Fuel | Heat gain (kJ) | CO2 (grams) |
| Coal | 32,8 | 3,66 |
| Petrol | 47,8 | 3,08 |
| Methane (natural gas) | 55,6 | 2,74 |
The burning of fossil fuels is the main source of the increase in greenhouse gas concentrations in the Earth's atmosphere and thus of man-made globalwarming. In order to avoid serious consequences of global warming, the fossil energy reserves known today may only be partially used. If the two-degree target is to be achieved with a probability of more than 50%, a maximum of between 870 and 1,240 gigatonnes (billion tonnes) of carbon dioxide may be released in the period 2011 to 2050, based on IPCC data. Converted to reserves, this means that in a global context about 30% of oil reserves, 50% of natural gas reserves and more than 80% of coal reserves must not be burned. If current emissions were maintained, the remaining carbon budget would be used up in 20-30 years. From the start of industrialisation until 2015, around 530 billion tonnes of carbon have already been released by fossil fuels, of which around just under half remained in the atmosphere and a good quarter each was absorbed by oceans and terrestrial ecosystems.
On the other hand, a complete burning of fossil energy resources, conservatively estimated at 5 trillion tons of carbon, would lead to an average warming of the Arctic by about 14.7 to 19.5 °C and a global temperature increase of about 6.4 to 9.5 °C, with very strong negative impacts on ecosystems, human health, agriculture, the economy, and so on. If unconventional resources were burned in addition to conventional ones, the concentration of carbon dioxide in the Earth's atmosphere could rise to about 5000 ppm by the year 2400. Such a scenario would lead to an increase in temperature to levels not seen for at least 420 million years. In addition, the Antarctic ice sheet would melt almost completely, causing sea levels to rise by about 58 m even without including the Greenland ice sheet.
Fossil energy sources
Hydrocarbons
Petroleum
→ Main article: Petroleum
Crude oil is a homogeneous and lipophilic mixture of substances, mainly consisting of long-chain hydrocarbons, deposited in the earth's crust. It originated from dead microorganisms (mostly unicellular algae), which were deposited on the seabed in oxygen-free water as mud enriched in organic compounds. Because the environment is subject to sometimes drastic changes over geological periods, at some point the sedimentation of the algal mud stopped and it was overlain by other sediments.
The loading pressure of the overlying sedimentary layers caused the mud to compaction to a fine-grained sedimentary rock. Continuous subsidence of the regional earth's crust - which enabled the deposition of further layers on top of the mud - brought the organic-rich rock into increasingly deeper crustal regions. There, increased temperatures prevailed as a result of the geothermal gradient. Under these conditions, the solid organic compounds in the rock were gradually converted into liquid and gaseous hydrocarbons. Since these hydrocarbons are relatively mobile and also have a lower density than the surrounding rock, they migrated towards the Earth's surface in permeable rock. Where impermeable rock layers effectively impeded their ascent, they accumulated in the permeable rock and formed reservoirs, with the gaseous hydrocarbons (mainly methane) usually accumulating as natural gas above the liquid petroleum.
With more than 17,000 components, untreated crude oil is one of the most complex mixtures of organic compounds naturally occurring on earth. The uses of crude oil are very diverse: in addition to combustion for heating purposes and in transport, it is the starting point for petrochemicals and thus one of the most important industrial raw materials.
Natural gas
→ Main article: Natural gas
Natural gas was formed in a similar way to crude oil and often occurs in association with it. It consists mainly of methane, but its exact composition varies. Due to its high methane content, unburned natural gas is a potent greenhouse gas. When processed, however, it burns cleaner and is more climate-friendly than other fossil fuels. However, extraction, transport and processing also contribute to the release of the greenhouse gases methane and carbon dioxide. Natural gas is used in particular for heat and power generation and as a raw material in the chemical industry.
Coal
→ Main article: Coal
Coal (from Old German kolo = "coal") is a black or brownish-black, solid biogenic sedimentary rock that is more than 50 percent carbon by weight and more than 70 percent carbon by volume.
Coal is a source of energy and is used by humans as a fossil fuel. It is produced from plant remains that rot in the absence of air - e.g. at the bottom of swamps and bogs - and are exposed to increased pressures and temperatures after sinking into deeper areas of the earth's upper crust.
Hard coal is considered to be a higher quality coal, as it is very dense and pure, i.e. contains very little foreign matter. The calorific value of hard coal is correspondingly high. Hard coal, like crude oil, is therefore also called black gold. Lignite, which is less dense and contains a greater proportion of sulphur, is of lower quality; its calorific value is significantly lower, which is why burning lignite is the most carbon dioxide-intensive way of generating electricity.
Coal was the first fossil fuel to be used on a large scale. Its intensive use emerged in England in the 16th century. However, coal represented only a small fraction of the energy consumed in Europe until the middle of the 19th century. Its relative share in the energy mix then rose sharply until the middle of the 20th century, only to be pushed back again somewhat by oil and gas later on.
Peat
→ Main article: Peat
Peat is the first stage of carbonization. It is formed under exclusion of air in near-surface and drying waters. It is easily combustible in its dried state. For this reason, it is often mined in peatlands, which ecologists view critically. Peat was mainly used as a fuel at the beginning of industrialisation. Around 1880 peat was also used for firing in the iron and steel industry. Since the beginning of the 20th century, some countries, especially in Central Europe until the end of the 1970s, have operated larger peat-fired power plants. Finland uses the highest proportion of peat as an energy source in the world, 51 percent, with it contributing 6-7 percent to the country's primary energy and 20 percent to greenhouse gas emissions.
Questions and Answers
Q: What are fossil fuels?
A: Fossil fuels are fuels that come from old life forms that decomposed over a long period of time.
Q: What are the three most important fossil fuels?
A: The three most important fossil fuels are coal, petroleum, and natural gas.
Q: What is oil and gas made up of?
A: Oil and gas are hydrocarbons (molecules that have only hydrogen and carbon in them). Coal is mostly carbon.
Q: How do we get these fossil fuels?
A: These fuels are called fossil fuels because they are dug up from underground. Coal mining digs up solid fuel; gas and oil wells bring up liquid fuel.
Q: When did people start using fossil fuel?
A: Fossil fuel was not much used until the Middle Ages. Coal became the main kind of fuel with the Industrial Revolution.
Q: Is coal mostly composed of carbon?
A Yes, coal is mostly composed of carbon.
Search within the encyclopedia