Amino acid
Amino acids (AS), uncommonly also called aminocarboxylic acids, outdated amido acids, are chemical compounds with a nitrogen (N) containing amino group and a carbon (C) and oxygen (O) containing carboxylic acid group. Amino acids are found in all living things. They are the building blocks of proteins (albumen) and are released when proteins are broken down (proteolysis). An organism cannot produce essential amino acids itself, so they must be ingested with food.
The class of amino acids includes organic compounds that contain at least one amino group (-NH2 or substituted -NR2) and one carboxy group (-COOH) as functional groups, i.e. they have structural features of amines and carboxylic acids. Chemically, they can be distinguished according to the position of their amino group relative to the carboxyl group - if the amino group at the Cα atom is immediately adjacent to the terminal carboxyl group, this is called α-terminal and is referred to as α-amino acids.
Selected α-amino acids are the natural building blocks of proteins. They are linked together to form chains by the carboxy group of one amino acid forming a peptide bond with the amino group of the next. The amino acids thus linked to form a polymer differ in their side chains and together determine the shape with which the polypeptide then unfolds in the aqueous environment to form the native protein. This biosynthesis of proteins takes place in all cells at the ribosomes according to genetic information, which is present in the form of mRNA.
The base sequence of the mRNA encodes the amino acid sequence in triplets, whereby each base triplet represents a codon that stands for a specific proteinogenic amino acid. The amino acids hereby indicated as building blocks for the formation of proteins in a specific sequence form the proteins. In humans, there are 21 different proteinogenic amino acids, including selenocysteine in addition to the standard 20 (canonical) amino acids. After translation, the side chains of some amino acids incorporated in the protein can still be modified.
However, the spectrum of amino acids goes far beyond these twenty or so proteinogenic ones. So far, more than 400 non-proteinogenic naturally occurring amino acids are known that have biological functions. The comparatively rare D-amino acids represent a special group. The variety of synthetically produced and theoretically possible amino acids is even greater.
Some amino acids play a special role as neurotransmitters, as do various degradation products of amino acids; biogenic amines not only occur as messengers in the nervous system, but also develop a variety of physiological effects in the organism as hormones and tissue mediators.
The simplest amino acid, glycine, has been detected not only on Earth but also on comets, meteorites and in gas clouds in interstellar space.
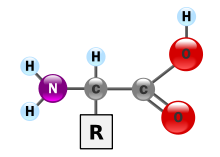
Basic structure of α-amino acids (residue R is a H atom in the case of glycine).
History
The first amino acid was isolated in 1805 in the Paris laboratory of Louis-Nicolas Vauquelin and his student Pierre Jean Robiquet from the juice of asparagus (Asparagus officinalis) and was subsequently named asparagine. The last of the common protein-building amino acids, threonine was discovered in fibrin in 1931 and its structure clarified by William Rose in 1935. Rose had found out by experiments with different feeds that the 19 amino acids discovered so far were not sufficient as an additive. He also established the essentiality of other amino acids and determined the minimum daily dose required for optimal growth.
In the period between 1805 and 1935, many of the chemists and pharmacists known at the time were involved in isolating amino acids for the first time and clarifying their structure. Emil Fischer, for example, to whom the Fischer projection also goes back, succeeded in finally elucidating the structure of serine (1901), lysine (1902), valine (1906) and cysteine (1908). Albrecht Kossel (1896 histidine from sturgeon sperm), Richard Willstätter (1900 proline via synthesis) and Frederick Hopkins (1901 tryptophan from casein) also later became Nobel Prize winners. The German chemist Ernst Schulze isolated three amino acids for the first time - glutamine from beets in 1877, phenylalanine in 1881 and arginine from lupins in 1886 - and was involved in the structural elucidation of other amino acids. Heinrich Ritthausen had previously obtained glutamic acid from cereal protein, gluten, in crystalline form in 1866. In 1872, Wilhelm Dittmar clarified the structure of glutamine and glutamic acid, whose salts are glutamates.
As early as 1810, William Hyde Wollaston discovered the sulfur-containing cystine as "cystic oxide" in bladder stones, but it was not until 1884 that Eugen Baumann discovered the monomeric cysteine. In 1819 Henri Braconnot separated glycine from glue and Joseph Louis Proust separated leucine from cereals. Eugen von Gorup-Besánez isolated valine from pancreatic juice in 1856. As early as 1846, Justus von Liebig was able to separate tyrosine from casein for the first time, the structure of which was clarified by Ludwig von Barth in 1869. In the hydrolysate of casein, Edmund Drechsel also discovered lysine in 1889 and later John Howard Mueller in 1922 discovered the sulphur-containing methionine as the 19th amino acid, whose structural formula was given by George Barger and Philip Coine in 1928. In molasses, Felix Ehrlich had already found isoleucine, a structural isomer of leucine, as the 18th amino acid in 1903.
Friedrich Wöhler, whose syntheses in the 1820s opened up the field of biochemistry, did not discover any amino acid, but three of his students were involved, in addition to the aforementioned Gorup-Besánez and Schulze, also Georg Städeler (1863 serine from raw silk). 18 of the 20 amino acids discovered were isolated from plant or animal material, only the two amino acids alanine (1850 Adolph Strecker) and proline (Willstätter) were obtained by organic synthesis. While the analysis of the material composition up to the sum formula could be well accomplished with the methods of the time, the structural formula of many amino acids could often only be finally elucidated by partial steps of the synthesis, which was sometimes only achieved years later. The structure of asparagine and that of aspartic acid was not clarified by Hermann Kolbe until 1862, 57 years after the first description.
Amino acids owe their generic names to two functional groups, their individual names sometimes to a bright appearance (e.g. arginine, leucine), a sweet taste (e.g. glycine) or the material in which they were found (e.g. asparagine, cysteine, serine, tyrosine), features of the chemical structure (e.g. proline, valine, isoleucine) or both (e.g. glucine). e.g. asparagine, cysteine, serine, tyrosine), features of the chemical structure (e.g. proline, valine, isoleucine) or both (e.g. glutamine, glutamic acid) and sometimes also the reactants of their synthesis (e.g. alanine).
The fact that proteins are built up as chains of amino acids linked by peptide bonds was first proposed simultaneously and independently of each other by both Emil Fischer and Franz Hofmeister at the Assembly of German Natural Scientists and Physicians in Karlsbad in 1902 (Hofmeister-Fischer theory).
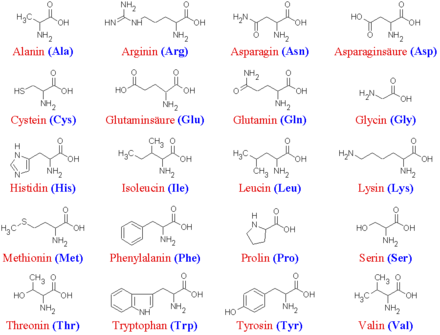
Simplified structural formulas of 20 natural α-amino acids, without configuration information.
Structure
|
Carbamic acid |
Amino acids consist of at least two carbon atoms. The unstable carbamic acid has only one carbon atom and is therefore not an amino acid, but a carbonic acid amide. Amino acids can be divided into classes according to the carbon atom at which the amino group is located relative to the carboxy group. If more than one amino group is present in the molecule, the carbon atom whose amino group is closest to the carboxy carbon determines which class of amino acid it is.
| General structure of amino acids |
|
α-amino acid |
|
β-amino acid |
|
γ-amino acid |
- α-Amino acids: The amino group of α-amino acids is located at the second carbon atom, including the carboxy carbon atom. The counting always starts with the carboxy-carbon. Therefore, the IUPAC designation is 2-aminocarboxylic acids. The simplest representative of the α-amino acids is the proteinogenic amino acid glycine. All proteinogenic amino acids are α-amino acids.
The term amino acids often refers to a specific group of α-amino acids consisting mainly of L-α-amino acids: the proteinogenic amino acids. These are the building blocks of all proteins of all life on earth and, along with nucleic acids, the basic building blocks of life.
- β-Amino acids: The amino group of β-amino acids is located on the third carbon atom (counting the carboxy carbon atom). The IUPAC designation is 3-aminocarboxylic acids. The simplest representative is β-alanine.
- γ-Amino acids: The amino group of γ-amino acids is located at the fourth carbon atom (counting the carboxy carbon atom). The IUPAC designation is 4-aminocarboxylic acids. The simplest representative is γ-aminobutyric acid (GABA).
The designation of other classes of amino acids follows the same scheme.
The amino acids of a class are distinguished by their side chain R. If the side chain R is different from the other substituents located on the carbon with the amino group, then a stereocenter is located here and two enantiomers exist of the corresponding amino acid. If the side chain R itself contains further stereocentres, diastereomers also result and the number of possible stereoisomers increases in proportion to the number of further stereocentres. There are four stereoisomers of amino acids with two differently substituted stereocentres.
Questions and Answers
Q: What are amino acids?
A: Amino acids are molecules that have both amine (NH2+R) and carboxyl (C=O) functional groups, and they are the building blocks of proteins.
Q: How many "standard" amino acids exist in eukaryotes?
A: In eukaryotes, there are 20 "standard" amino acids out of which almost all proteins are made.
Q: What is the general formula for alpha-amino acids?
A: The general formula for alpha-amino acids is H2NCHRCOOH, where R is one of many side groups.
Q: What does biochemistry refer to when it mentions amino acids?
A: In biochemistry, the term 'amino acid' refers to alpha-amino acids with the general formula H2NCHRCOOH, where R is one of many side groups.
Q: How do proteins get their structure?
A: Proteins get their structure from the combination of different types of amino acids.
Q: What role do amine and carboxyl functional groups play in an amino acid molecule?
A: Amine and carboxyl functional groups make up an amino acid molecule; they provide a nitrogen atom as well as a carbon atom that can form bonds with other molecules.
Search within the encyclopedia



