Enthalpy
The enthalpy 




It has the dimension of energy and is measured in the unit joule.
Enthalpy is an extensive quantity: The enthalpy of a total system is the sum of the enthalpies of the subsystems.
The molar enthalpy (unit: J/mol) is the enthalpy related to the amount of substance

The specific enthalpy (unit: J/kg) is the enthalpy related to the mass

The molar and specific enthalpies are intensive quantities: If two identical subsystems have the same molar or specific enthalpy, then the total system formed from them also has this molar or specific enthalpy.
The enthalpy 



The practical usefulness of the enthalpy calculation parameter is based on the fact that the change in the enthalpy of a system caused by a process is
by the simpler expression
if the process takes place at constant pressure (isobaric, 




Numerous physical and chemical processes take place at constant pressure. This is often the case, for example, with phase transitions or chemical reactions, especially (but not only) when they take place under atmospheric pressure. The enthalpy is then a suitable quantity for describing the heat conversion of these processes.
In theoretical thermodynamics, enthalpy is a fundamental function from which the entire thermodynamic information about the system can be derived. However, the prerequisite is that it is a function of the variables entropy 


The enthalpy is a Legendre transform of the internal energy. The internal energy is also a fundamental function if it is expressed as a function of its natural variables 







To be distinguished from enthalpy is free enthalpy or Gibbs energy, the Legendre transformation of enthalpy after entropy.

The enthalpy of reaction of the combustion of alcohol in air is negative. It is therefore an exothermic reaction in which heat is released into the environment.

The melting enthalpy is the amount of energy required to melt the ice at constant pressure. It is extracted from the environment and cools the drink in the process.
Enthalpy in isobaric physical and chemical processes
Numerous processes from physics (e.g. phase transitions) or from chemistry (e.g. chemical reactions) take place under constant pressure. In these cases, the enthalpy allows a simple description and calculation of the heat conversion.
Standard enthalpy of formation
As already mentioned, any process that connects the two states can be used to calculate the enthalpy difference between two states. For example, in a chemical reaction, one can mentally break down the starting materials into their elements and reassemble them into the product materials. The enthalpy to be expended or dissipated in each case is the so-called enthalpy of formation of the substance in question. The enthalpies of formation depend on temperature and pressure. The enthalpies of formation that are converted under standard conditions are the standard enthalpies of formation.
The molar standard enthalpy of formation (usually abbreviated to standard enthalpy of formation) is the enthalpy that is released (negative sign) or required (positive sign) for the formation of one mole of a substance from the allotropically most stable form of the pure elements under standard conditions (100 kPa and 25 °C). It is usually expressed in kilojoules per mole. The quantity is formulated Δ 



If it is negative, it is an exothermic reaction and energy is released during the formation of the substance from the elements (heat of formation). If, on the other hand, it is positive, it is an endothermic reaction and energy must be expended to form the substance from its initial elements. Strongly negative values of the standard enthalpy of formation are a characteristic of chemically particularly stable compounds (i.e. a lot of energy is released during their formation and a lot of energy must be expended to destroy the bonds). The standard enthalpy of formation of the chemical elements in their most stable state (H2, He, Li, ...) is set to 0 kJ/mol by definition.
If the standard enthalpies of formation of the substances involved in a chemical reaction are known, the enthalpy of reaction of this reaction under standard conditions can be easily calculated. It is the difference between the standard enthalpies of formation of the reaction products on the one hand and the starting materials (reactants; "reactants") on the other (Hess theorem):
All values refer to thermodynamic equilibrium, otherwise the temperature would not be defined.
Conversely, the standard enthalpy of formation can be determined with the help of Hess' theorem from enthalpies of reactions in which the respective substance participates as a reactant or product. If no experimental data are available, an estimation of the standard enthalpies of formation can also be estimated using group contribution methods. The increment method according to Benson is particularly suitable for this.
Inorganic substances
| Chemical formula | Fabric |
| Source |
| H2O (g) | Water (gaseous) | −241,83 | |
| H2O (l) | Water (liquid) | −285,83 | |
| CO2 (g) | Carbon dioxide | −393,50 | |
| NH3 (g) | Ammonia | −45,94 |
Organic substances
| Sum formula | Fabric |
| Source |
| CH4 (g) | Methane | −74,87 | |
| C2H4 (g) | Ethylene | +52,47 | |
| C2H6 (g) | Ethan | −83,8 |
Enthalpy in physics (thermodynamics)
In a narrower sense, thermodynamics describes only the intermolecular forces, i.e. the energetic relationships (phase states or their changes) between the individual molecules of a substance.
Enthalpy of evaporation / enthalpy of condensation
→ Main article: Heat of evaporation
The molar enthalpy of vapourisation Δ 
The molar enthalpy of condensation Δ 
The enthalpy of vapourisation decreases with increasing temperature and becomes zero when the critical point is reached, as it is no longer possible to distinguish between liquid and gas there. As a rule, in tabular works, enthalpy of vaporisation data are either related to 25 °C or tabulated for the various temperature-pressure combinations along the boiling point curve, it always applies Δ 
For mixtures or solutions of substances, the enthalpies add up in the ratio of their mixture proportions.
If no enthalpy of vapourisation values are available for a substance, these can be calculated for any temperature with the help of the Clausius-Clapeyron equation if the temperature dependence of the vapour pressure curve is known at the temperature under consideration.
In rare cases, values for enthalpies of vaporisation were tabulated. The enthalpy of vaporisation can always be derived from the thermodynamic data by difference formation if standard enthalpy of formation values for the liquid and gaseous state of aggregation are known, e.g. for water, carbon disulphide, methanol, ethanol, formic acid, acetic acid, bromine in the table above.
Take table salt, for example:
- Evaporation enthalpy ∆VH = +170 kJ/mol (at 1465 °C, table value)
- For the practical calculation of the heat of evaporation, the following table values for the enthalpy of formation are used:
| NaCl (melt) | | NaCl (g) |
|
| -386 kJ/mol |
| -182 kJ/mol | Formation enthalpies (25 °C) |
|
|
| ||
The difference between the two values 170 and 204 is within the usual range.
Enthalpy of sublimation
Sublimation describes the transition of a solid into the gas phase bypassing the liquid melt phase (technical application in freeze-drying). The enthalpy of sublimation is sometimes listed in tables. In principle, the enthalpy of fusion and the enthalpy of evaporation may also be combined at the same reference temperature:
Enthalpy of sublimation = enthalpy of fusion + enthalpy of evaporation.
The enthalpy of sublimation should not be confused with the heat of sublimation. This corresponds to the sum of the heat of fusion and the heat of evaporation.
The enthalpy of sublimation can always be derived from the thermodynamic data if standard enthalpy of formation values for the solid and gaseous states of aggregation are known.
- Sublimation enthalpy of common salt: 211 kJ/mol (25 °C, table value)
- Calculation:
| NaCl (s) | | NaCl (g) |
|
| -411 kJ/mol |
| -182 kJ/mol | Formation enthalpies (25 °C) |
|
|
| ||
Note: The example shows that in principle one can also calculate processes that are hardly feasible in practice. The "sublimation enthalpy of elemental carbon" was "determined" in this way.
| Sublimation enthalpies of some substances | ||||
| Fabric sign | Fabrics | Enthalpy of sublimation (kJ/mol) | Standard enthalpy of formation (solid) (KJ/mol) | Standard enthalpy of formation (gas) (KJ/mol) |
| Na | Sodium | 108,7 | 0,0 | 108,7 |
| K | Potassium | 90,0 | 0,0 | 90,0 |
| Br2 | Bromine | 30,71 | 0,0 | 30,71 |
| Li | Lithium | 155,10 | 0,0 | 155,10 |
| I2 | Iodine | 62,4 | ||
| C10H8 | Naphthalene | 72,6 | ||
| CO2 | Carbon dioxide | 26,1 | ||
Enthalpy of fusion / enthalpy of crystallisation
After heating a solid substance to its melting point temperature, heat of fusion is absorbed at this temperature without the temperature rising any further. This form of heat is called latent heat because it does not cause a change in temperature. In the case of ionic solids, the phase transformation solid/liquid results in salt melts with easily movable ions (technical application in fused-salt electrolysis). Table salt melts at 800 °C.
Melting halpies are rarely recorded in tables.
The enthalpy of fusion can always be derived from the thermodynamic data if standard enthalpy of formation values for the solid and liquid states of aggregation are known.
- Melting enthalpy of common salt: 28; 30.2 kJ/mol (800 °C, table values)
- For practical calculation of the heat of fusion, the following table values are used:
| NaCl (s) | | NaCl (melt) |
|
|
|
| -411 kJ/mol |
| -386 kJ/mol |
|
| Formation enthalpies (25 °C) |
| NaCl (s) | | Na+ (melt) | + | Cl- (melt) |
|
| -411 kJ/mol |
| approx. -215 kJ/mol |
| approx. -170 kJ/mol | Formation enthalpies (25 °C) |
|
|
| ||||
In the reversal of this process, crystallisation from the melt, the ions of a salt can combine directly to form their solid crystal lattice. During the precipitation of common salt crystals from the melt, -25.2 kJ/mol enthalpy of crystallisation (or 29±1 kJ/mol at 800 °C) is released.
Experience shows that "supercooled molten salts" can release considerable amounts of heat due to spontaneous crystallisation. (Application: heating pad).
Lattice enthalpy
According to a common definition, the lattice energy is the energy that must be expended in a vacuum (i.e. at external pressure 




The lattice enthalpy of NaCl is composed as follows:
| NaCl (solid) | | Na+ (g) | + | Cl- (g) |
|
| -411 kJ/mol |
| 611 kJ/mol |
| -244 kJ/mol | Formation enthalpies (25 °C) |
|
|
| ||||
Comparison: This is approximately twice the necessary energy that would be released in the strongly exothermic reaction of sodium metal and chlorine gas. The formation of gaseous ions is therefore extremely endothermic.
The lattice enthalpy ΔH0L depends on the size and charge of the ions involved and is always positive in this type of definition, otherwise the lattice would not be stable. Aluminium oxide Al2O3 (Al3+ and O2-) has a very high lattice enthalpy of 15157 kJ/mol. The high lattice enthalpy is exploited in aluminothermic processes; these include aluminothermic welding and the preparation of elements from their oxides and aluminium by means of aluminothermy. In the latter case, the high lattice enthalpy of the aluminium oxide is a main driving force for the reaction, as it is directly reflected in the Gibbs energy.
The lattice energy is often also defined as the enthalpy of reaction in the formation of the solid salt lattice starting from ions in the gas phase. If the lattice energy is defined in this way, the process is exothermic and the associated enthalpy change must be given as negative. The lattice enthalpy of aluminium oxide would then be -15157 kJ/mol, for example.
The lattice enthalpy depends on the size of the ions involved: The larger the ions, the smaller the lattice energy released, since the attractive forces decrease with increasing distance of the positive nuclei from the negative electron shell of the bonding partner.
Examples: Molar lattice enthalpy of alkali fluorides at 25 °C in kJ/mol:
| Name | Formula | Ion radius of the monovalent alkali metal cations | Lattice enthalpy in kJ per mol |
| Lithium fluoride | LiF | 74 | 1039 |
| Sodium fluoride | NaF | 102 | 920 |
| Potassium fluoride | KF | 138 | 816 |
| Rubidium fluoride | RbF | 149 | 780 |
| Caesium fluoride | CsF | 170 | 749 |
On the other hand, the lattice energy depends on the electrical charge of the ions involved: The greater the charges, the greater the attractive forces and the greater the lattice energy.
Examples: molar lattice enthalpy at 25 °C in kJ per mol (in the examples, the ion radius changes only slightly):
| Name | Formula | Cations | Anions | Lattice enthalpy in kJ per mol |
| Sodium chloride | NaCl | Na+ | Cl- | 780 |
| Sodium sulphide | Na2S | Na+ | S2− | 2207 |
| Magnesium chloride | MgCl2 | Mg2+ | Cl- | 2502 |
| Magnesium sulphide | MgS | Mg2+ | S2− | 3360 |
For a similar effect with graphite under neutron irradiation, see Wigner energy.
Solvation enthalpy, hydration enthalpy
It indicates the energy released when gaseous ions attach to solvents, i.e. form solvated ions. For the most frequent case of solvent = water, one speaks of enthalpy of hydration.
| Na+ (g) | | Na+ (hydrated) |
|
| 611 kJ/mol |
| -240 kJ/mol | Formation enthalpies (25 °C) |
|
|
| ||
|
| |||
| Cl- (g) | | Cl- (hydrated) |
|
| -244 kJ/mol |
| -167 kJ/mol | Formation enthalpies (25 °C) |
|
|
| ||
The hydration enthalpy of the gaseous ions of common salt is strongly exothermic with -774 kJ/mol overall.
Enthalpy of solution / enthalpy of crystallisation
The enthalpy of solution of salts involves 1) the separation of the ion lattice into single ions and 2) the solvation of the single ions. Sub-step 1) is very strongly endothermic, sub-step 2) very strongly exothermic.
Solution enthalpy = lattice enthalpy + solvation enthalpy.
Solution enthalpy NaCl in water = (+778 kJ/mol) + (-851+77 kJ/mol) = +4 kJ/mol (25 °C).
This value is in good agreement with table works +3.89 kJ/mol for the saline heat of dissolution. Thus, a very slight cooling of the solution occurs during dissolution.
Of course, for practical calculations of the heat of solution one does not take the diversions via the lattice energy, but one calculates directly and with only a few table values (occasionally one finds the value (NaCl)hydrat. = -407 kJ/mol instead of the individual ions):
| NaCl (s) | | NaCl (hydrated) |
|
|
|
| -411 kJ/mol |
| -407 kJ/mol |
|
| Formation enthalpies (25 °C) |
| NaCl (s) | | Na+ (hydrated) | + | Cl- (hydrated) |
|
| -411 kJ/mol |
| -240 kJ/mol |
| -167 kJ/mol | Formation enthalpies (25 °C) |
|
|
| ||||
The enthalpy of solvation can always be derived from the thermodynamic data if one finds standard enthalpy of formation values for the solid and dissolved state of aggregation, e.g. formic acid and carbon dioxide in the table above. It applies to "infinite dilution".
When this process is reversed, crystallisation from solution, the dissolved ions of a salt 1) release their solvate shell and 2) join together in a solid crystal lattice. During the precipitation of common salt crystals from water, -3.89 kJ/mol enthalpy of crystallisation is released.
Intermolecular enthalpy contributions
Differently strong interactions between the molecules are the reason why groups of substances have similar or very different enthalpies of sublimation.
- Weak contributions are provided by London forces, dipole-dipole interactions and ion-dipole interactions with 1-15 kJ/mol binding. They are summarised as Van der Waals interactions. See the melting and evaporation enthalpies of hydrogen, carbon monoxide and methane as examples.
- Stronger contributions are provided by hydrogen bonds with 20-40 kJ/mol bond (depending on polarisation). See as examples the enthalpies of melting and evaporation of water, methanol and formic acid and also enthalpies of hydration. Hydrogen bonds are also responsible for the fact that the boiling point of water is 100 °C, while that of hydrogen sulphide is only -83 °C (see boiling point anomaly).
- Very strong contributions are made by ion-ion interactions in crystals. In the crystal, sodium chloride does not consist of discrete NaCl molecules, but of an equal number of sodium cations and chloride anions arranged exactly in the crystal lattice according to Coulomb forces.
Enthalpy in chemistry (thermochemistry)
In a narrower sense, thermochemistry describes only the intramolecular forces, i.e. the energetic relationships between the individual atoms of a molecule. Covalent bonds contain approx. 150-1000 kJ/mol of binding energy, ionic bonds approx. five times this amount.
If the standard enthalpies of formation of reactants and products are known, a possible chemical reaction can be roughly balanced energetically. The most important question is often whether a process is endothermic or exothermic and to what extent.
Details such as evaporation, melting, solvation or crystallisation enthalpies can be used to specify partial steps within the chemical reaction in terms of energy. The energy content of the reaction can be specified.
Reaction enthalpy
→ Main article: Enthalpy of reaction
The enthalpy of reaction is the energy released or required when new chemical bonds are formed between the molecules of two substances. It depends on the reaction partners (reactants) and the type of chemical bond in the product. To calculate it, one compares the sum of the enthalpies of formation of the products with that of the reactants. The difference is the enthalpy of reaction, which can then be standardised by reference to the amount of substance in the product of interest:
| 2 Na (s) | + | Cl2 (g) | | 2 NaCl (s) |
|
| 2 × 0 kJ/mol |
| 0 kJ/mol |
| 2 × -411 kJ/mol | Formation enthalpies (25 °C) |
|
| |||||
The reaction is therefore exothermic. Dividing by the amount of substance obtained, in this case 2 mol sodium chloride, yields its molar enthalpy of formation of -411 kJ/mol NaCl (25 °C) (in this example, however, already assumed at the beginning).
Standard enthalpy of combustion
Combustion is also a chemical reaction. The enthalpy of reaction of the combustion reaction or the standard enthalpy of combustion of a substance is the enthalpy change that occurs when a substance burns completely under excess O2 (O2 overpressure) and standard conditions (101.325 kPa and 25 °C). By definition, this heat of combustion refers to the formation of gaseous carbon dioxide and liquid water (or N2) as end products; no gaseous water can form under excess oxygen pressure. It is designated ΔVH0 or ΔcH0 (c for combustion) and its absolute amount is identical to the calorific value Hs.
The following reaction is carried out in an autoclave tube with excess oxygen pressure:
| C3H8 (g) | + | 5 O2 (g) | | 3 CO2 (g) | + | 4 H2O (l) |
| -103.2 kJ/mol |
| 5 × 0 kJ/mol |
| 3 × -393.5 kJ/mol |
| 4 × -285.8 kJ/mol |

Division by the amount of substance used, in this case 1 mol propane, gives its molar enthalpy of combustion of -2.22 MJ/mol propane (25 °C)
The same reaction in an open burner flame; only gaseous combustion products are formed:
| C3H8 (g) | + | 5 O2 (g) | | 3 CO2 (g) | + | 4 H2O (g) |
| -103.2 kJ/mol |
| 5 × 0 kJ/mol |
| 3 × -393.5 kJ/mol |
| 4 × -241.8 kJ/mol |

Division by the amount of substance used, in this case 1 mol propane, gives its molar enthalpy of combustion of -2.04 MJ/mol propane (25 °C)
Advanced application
It is tedious to look up the standard enthalpies of formation of the reactants and products for each reaction, and in the correct state of aggregation. Moreover, with larger molecules one quickly encounters a "data vacuum". The following simplified considerations have proven themselves in practice:
- It is irrelevant whether one brominates a long-chain alkyl-substituted ethylene or ethylene itself, the heat of reaction per (C=C) double bond is largely the same.
- It is irrelevant whether one calculates a reaction completely in the liquid phase or completely in the gas phase, the heat of reaction hardly influences this.
- It is insignificant (some deviations) to calculate reactions carried out at 150 °C for 25 °C standard conditions. (The enthalpy of reaction can be calculated for any temperature if the temperature dependence of the molar heats of all reactants is known).
Therefore, one can calculate normal organic chemical conversions such as halogen additions, cycloadditions, esterifications with acids or anhydrides, hydrolyses etc. with the help of numerous tabulated increments for gaseous molecules according to Benson.
In the following example, the reaction energy of the bromine addition to ethylene is calculated with Benson increments and estimated for comparison from binding energies of involved bonds. (Note: Bonding energies are averaged dissociation energies, not standard enthalpies of formation!)
Bromine addition to an alkene, reaction enthalpy calculated with standard enthalpies of formation:
| H2C=CH2 | + | Br2 | | H2BrC-CBrH2 |
| 52 kJ/mol |
| 0 kJ/mol |
| -39 kJ/mol |

Bromine addition to an alkene, reaction enthalpy calculated with increments according to Benson:
| H2C=CH2 | + | Br2 | | H2BrC-CBrH2 |
| 2 Cd-(H)2: 2× +28.1 kJ/mol |
| 2 C-(H)2(Br)(C): 2× -22.6 kJ/mol | ||

Bromine addition to an alkene, reaction enthalpy estimated with binding energies:
| H2C=CH2 | + | Br2 | H2BrC-CBrH2 | |
| 4 C-H: 4×>455 kJ/mol |
| 4 C-H: 4× 380±50 kJ/mol | ||
| 1 C=C: 1× 614 kJ/mol |
| 1 C-C: 1× 348 kJ/mol | ||
| 1 Br-Br: 193 kJ/mol |
| 2 C-Br: 2× 260±30 kJ/mol | ||

The best estimation for reaction enthalpies is achieved with standard enthalpies of formation or increments according to Benson; when using "binding energies", the uncertainty of ±200 kJ/mol is much too high.
Technical applicability
The reaction enthalpies of organic reactions are in the range -160 to +100 kJ per mole of "reactive centres". All addition reactions with epoxides, anhydrides and halogens prove to be very strongly exothermic. These thermochemical observations make no statement about how quickly these reaction heats are released. They only make the statement that this heat is released by the end of the reaction. Each reaction increases its speed by a factor of two to three with a temperature increase of 10 K. Conversely, a twofold dilution of the reactants often means a halving of the reaction rate or heat output of the reaction. Calculated reaction enthalpies are used to calculate the temperature curve in a system of reactants and solvents via their heat capacities. Large-scale plants only have limited cooling capacities (-heat/time), this is often not taken into account in laboratory experiments.
Binding energy / dissociation energy
→ Main article: Binding energy (chemistry)
The binding energy or binding strength indicates the "stability" of the covalent bond. The determination is only possible directly for diatomically symmetrical molecules such as hydrogen or halogens. In these cases, the dissociation energy to form two identical radicals can be easily measured/calculated. For "element radicals", the standard enthalpy of formation of radicals is also called atomisation enthalpies.
In all other cases, values for the "binding energy" are only possible indirectly by comparing several dissociation energy measurements on homologous molecules. The values vary depending on the substitution pattern at the radical centres.
The standard enthalpy of formation of gaseous
- Bromine radicals includes the enthalpy of vaporisation (31 kJ/mol) necessary to convert liquid bromine into gaseous bromine.
- Iodine radicals includes the sublimation enthalpy (62 kJ/mol) necessary to convert crystalline iodine into gaseous iodine.
- carbon radicals is identical to the standard enthalpy of formation of gaseous carbon vapour.
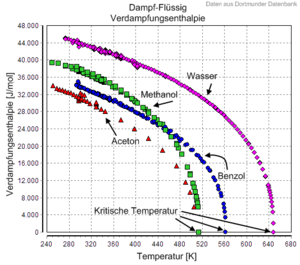
Temperature dependence of the enthalpy of vaporisation of water, methanol, benzene and acetone.
Thermodynamic properties of enthalpy
Enthalpy as a fundamental function
Consider a system whose properties are defined by the state variables entropy 




a fundamental function of the system. It describes the system completely; all thermodynamic properties of the system can be derived from it.
Often, however, these variables are inconvenient for practical work and one would prefer to have, for example, the temperature or the pressure in the variable list. In contrast to the usual procedure, a change of variable in the present case must not be done by a simple substitution, as otherwise information is lost. If, for example, the volume is to be 




this formulation would be equivalent to a partial differential equation for 


To change variables while preserving the complete information, a Legendre transformation must be performed. For example, if the variable list 

The Legendre transform 

The origin of the enthalpy from a Legendre transformation explains the additive term 
Fundamental functions that have the dimension energy are also called thermodynamic potentials. Enthalpy is therefore a thermodynamic potential.
Derivatives of enthalpy
If one starts from the internal energy as a function of its natural variables and forms its total differential, one obtains:

The partial derivatives occurring here are interpreted in thermodynamics as the definitions of temperature 


so that the differential can also be written as

The total differential of enthalpy as a function of its natural variables is on the one hand formally

and on the other hand, using their definition
so that from the comparison of the coefficients in the marked equations follows

and
and

The derivation simultaneously shows how the addition of the term 




The second of the marked equations is a "differential fundamental function", namely the differential enthalpy as a function of its natural variables:

Minimum enthalpy principle
According to the Second Law of Thermodynamics, a closed system takes as its equilibrium state among the attainable states the one that has the highest entropy at the given internal energy. From this maximum principle of entropy, a minimum principle of internal energy can be derived: If entropy is kept constant, a system adopts as its equilibrium state that state which has the lowest internal energy.
A similar minimum principle exists for enthalpy: A system that is kept at constant pressure assumes as its equilibrium state, of all the states that can be reached with this pressure, the one in which the enthalpy has the smallest possible value.
To prove this, consider a system whose pressure is kept at a constant value. Pressure control can be achieved, for example, by the system under consideration being in contact, via a movable adiabatic wall, with a second system that invariably has the desired pressure (in thermodynamic terms: a pressure reservoir). By moving the contact wall, the system under consideration can, if necessary, "exchange volume" with the pressure reservoir until it has brought its pressure back into line with that of the reservoir. The total system formed by the system under consideration and the pressure reservoir assumes the lowest possible internal energy 

Since only volume is exchanged with the reservoir according to its definition, the internal energy of the reservoir can only be changed by performing volume change work on it: 

When the partition is displaced, 

Since the pressure of the reservoir, 

The extremal properties of the function 







As can be shown, the second derivative of 
The minimum principle for the internal energy of the total system at constant entropy thus leads to the enthalpy of the system under consideration assuming a minimum on the subset of states with constant pressure 
If one wants to determine the state of equilibrium with the help of the (generally and always valid) entropy criterion, the maximum of the total entropy must be determined, i.e. the sum of the entropies of the system under investigation and its environment. Therefore, not only the change of the system entropy during a change of state must be considered, but also the entropy change that the system produces there through reaction on the environment. The enthalpy criterion is a reformulation of the entropy criterion, in which only properties of the system under consideration are taken into account and which automatically takes into account the retroactive effect on the environment (under isobaric conditions). When using the enthalpy criterion, the determination of the (isobaric) state of equilibrium can thus be limited to the observation of the system, which noticeably facilitates the investigations.
If the enthalpy is 





For a real physical or chemical process, the atmosphere can often serve as a pressure reservoir. Because of its large volume, its pressure does not change appreciably when a system performs volume change work on it. The conditions for the applicability of the minimum enthalpy principle are thus fulfilled if a system is adiabatically isolated from the environment (to prevent heat exchange with the environment and thus keep entropy constant) and coupled to the ambient atmospheric pressure via a movable piston or similar device (to keep the system pressure constant).
In laboratory practice, however, such adiabatic systems rarely occur. Chemical reactions usually do not take place in adiabatically insulated vessels, so that heat can be exchanged with the environment. The atmosphere then serves not only as a pressure reservoir but also as a heat reservoir: it keeps pressure and temperature constant. The thermodynamic potential that assumes a minimum under these conditions is the Gibbs energy 
Enthalpy and heat capacity
→ Main article: Specific heat capacity
If one adds the specific (i.e. related to the mass) amount of heat 


or

The specific heat capacity depends not only on the material but also on the process control. If the heat supply is isochoric (i.e. at constant volume), then the total amount of heat supplied contributes to the increase of the specific internal energy
and the process is described by the specific heat capacity at constant volume,

which is therefore the derivative of the specific internal energy according to the temperature at constant volume.
If the heat input is isobaric, then the specific heat input is equal to the increase in specific enthalpy 
and the process is described by the specific heat capacity at constant pressure,

which is therefore the derivative of the specific enthalpy according to the temperature at constant pressure.
Since to achieve a desired temperature increase in the isobaric case, the volume change work to be performed by the system must be additionally supplied, it is to be expected that the isobaric specific heat capacity of a substance will be greater than the isochoric one. A closer look must take into account that the expansion of the system usually also changes its internal energy. However, it can be shown that generally applies:

On the right side, the temperature 



is. In some cases, 

Temperature and pressure dependence of enthalpy
Tabulations of enthalpy usually refer to a specific temperature and pressure for reasons of space. If the enthalpy is to be determined for other conditions, formulas are desirable that allow the transition from the reference state to other temperatures and pressures. It is advantageous if only the knowledge of directly measurable quantities is required for the conversion.
For a closed system that is in equilibrium and in which no chemical reactions take place ( 

The differential 


With the help of the identities


and

follows

which can be calculated using the isobaric heat capacity 




For a finitely large change of state from the state 


If the change of state is associated with a phase transition, the relevant latent heat must also be taken into account.
The quantities 


Since enthalpy is a state variable, the integrals do not depend on the choice of path along which to integrate. A convenient choice of path is to first perform the temperature integral on a constant pressure path 











Alternatively, the integration can first be carried out on a constant temperature path from 










If the process is isobaric, 


See also
- Thermodynamic potential
- Gibbs Energy
- Isenthalp
- Dortmund database
- Group contribution methods (for the prediction of evaporation enthalpies)
Questions and Answers
Q: What is enthalpy?
A: Enthalpy is a concept used in science and engineering to calculate heat and work in a substance.
Q: Where does the name "enthalpy" come from?
A: The name "enthalpy" comes from the Greek word "enthalpos" (ενθαλπος), which means "to put heat into".
Q: Who made up the idea and word "enthalpy"?
A: The Dutch scientist Heike Kamerlingh Onnes made up the idea and word "enthalpy" in 1909.
Q: How does enthalpy relate to energy?
A: Enthalpy accounts for the energy used or released when a substance grows or shrinks, but it is not the same as energy.
Q: Why do scientists often calculate the change in enthalpy rather than the change in energy?
A: Scientists often calculate the change in enthalpy because it takes into account the energy used or released when a substance changes size.
Q: What happens when heat energy enters a system containing a fixed amount of gas?
A: When heat energy enters a system containing a fixed amount of gas, it can either increase the internal energy of the gas or lead to some work being done to expand the system.
Q: How does enthalpy take into account the factors of pressure and volume?
A: Enthalpy takes into account the factors of pressure and volume by considering the work done, which is the product of pressures and volume.
Search within the encyclopedia
















