Electron
![]()
This article is about the elementary particle. Further meanings under Elektron (Begriffsklärung).
The electron [ˈeːlɛktrɔn, eˈlɛk-, elɛkˈtroːn] (from ancient Greek ἤλεκτρον élektron "amber", on which electricity was studied in antiquity; coined by Stoney and Helmholtz in 1874) is a negatively charged elementary particle. Its symbol is e-. The alternative name negatron (from negative charge and electron) is hardly used any more and is at best common in beta spectroscopy.
The electrons bound in an atom or ion form its electron shell. The entire chemistry is essentially based on the properties and interactions of these bound electrons. In metals, a part of the electrons is freely movable and causes the high electrical conductivity of metallic conductors. This is the basis of electrical engineering and electronics. In semiconductors, the number of mobile electrons and thus the electrical conductivity can be easily influenced, both by the production of the material and later by external influences such as temperature, electrical voltage, incidence of light etc.. This is the basis of semiconductor electronics. Electrons can escape from any material when it is strongly heated or when a strong electric field is applied (glow emission, field emission). As free electrons, they can then be formed into an electron beam in a vacuum by further acceleration and focusing. This has enabled the development of cathode ray tubes (CRTs) for oscilloscopes, televisions and computer monitors. Other applications of free electrons include the X-ray tube, the electron microscope, electron beam welding, basic physics research using particle accelerators and the generation of synchrotron radiation for research and technical purposes.
During the beta-minus decay of an atomic nucleus, an electron is newly produced and emitted.
The experimental proof of the electron succeeded for the first time Emil Wiechert in the year 1897 and a little later Joseph John Thomson.
History of the discovery of the electron
The concept of a smallest, indivisible quantity of electric charge was proposed on several occasions around the middle of the 19th century, including by Richard Laming, Wilhelm Weber and Hermann von Helmholtz.
In 1874, George Johnstone Stoney proposed the existence of electric charge carriers associated with atoms. Starting from electrolysis, he estimated the magnitude of the electron charge, but obtained a value too low by a factor of about 20. At the British Association meeting in Belfast, he proposed that the elementary charge be used as another fundamental constant of nature, along with the gravitational constant and the speed of light, as the basis of physical measurement systems. Together with Helmholtz, Stoney also coined the name electron for the "atom of electricity".
In 1897, Emil Wiechert found that the cathode radiation consists of negatively charged particles that are much lighter than an atom, but then stopped his research on this. In the same year, Joseph John Thomson determined the mass of the particles (he first called them corpuscules) more precisely and was able to prove that they are always the same particles, regardless of the cathode material and the residual gas in the cathode ray tube. During this time, it was demonstrated by means of the Zeeman effect that these particles also occur in the atom and cause light emission there. Thus the electron was identified as an elementary particle.
The elementary charge was measured in 1909 by Robert Millikan.
Properties
The electron is the lightest of the electrically charged elementary particles. If the conservation laws for charge and energy apply - which corresponds to all physical experience - electrons must therefore be stable. In fact, there is no experimental evidence of electron decay so far.
The electron belongs to the leptons and, like all leptons, has a spin (more precisely: spin quantum number) of 1/2. As a particle with a half-integer spin, it belongs to the class of fermions and is therefore subject in particular to the Pauli principle. Its antiparticle is the positron, symbol e+, with which it has all the same properties except for its electric charge.
Some of the basic properties of the electron listed in the table above are linked by the magnetic moment of the electron spin:

Where μ 






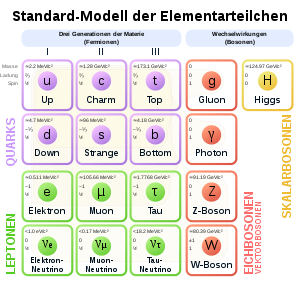
Standard model of elementary particles: the 12 fundamental fermions and 5 fundamental bosons; the electron ranks among the leptons
Questions and Answers
Q: What is an electron?
A: An electron is a very small piece of matter, and it is a subatomic particle. It cannot be broken down into anything smaller and has a negative electric charge.
Q: Who discovered the electron?
A: The electron was discovered by J.J. Thomson in 1897.
Q: How much mass does an electron have?
A: Electrons have very little mass, or weight, so very little energy is needed to move them fast.
Q: What type of interactions do electrons take part in?
A: Electrons take part in gravitational, electromagnetic and weak interactions. The electromagnetic force is strongest in common situations.
Q: How do electrons interact with each other?
A: Electrons repel from each other because they have the same electric charge, but they are attracted to protons because they have opposite electric charges.
Q: What powers televisions, motors, mobile phones and many other things?
A: The electricity that powers these devices is actually many electrons moving through wires or other conductors.
Search within the encyclopedia