Curium
![]()
The title of this article is ambiguous. For other meanings, see Curium (disambiguation).
Curium is an artificially produced chemical element with the element symbol Cm and the atomic number 96. In the periodic table it is in the actinide group (7th period, f-block) and belongs to the transuranics. Curium was named after the researchers Marie Curie and Pierre Curie.
Curium is a radioactive, silvery-white metal of great hardness. It is formed in nuclear reactors, a ton of spent nuclear fuel contains an average of about 20 grams.
Curium was first produced from the lighter element plutonium in the summer of 1944, and the discovery was not initially made public. It was not until an American radio program for children that the discoverer Glenn T. Seaborg, as a guest on the program, revealed its existence to the public by answering a young listener's question in the affirmative as to whether new elements had been discovered.
Curium is a strong α-emitter; it is occasionally used in radionuclide batteries because of the very large heat generated during decay. It is also used to produce 238Pu for low gamma-ray radionuclide batteries, for example in cardiac pacemakers. The element can further be used as a starting material for the generation of higher transuranics and transactinoids. It is also used as an α-ray source in X-ray spectrometers, with which the Mars rovers Sojourner, Spirit and Opportunity, among others, chemically analyse rocks on the surface of the planet Mars. The lander Philae of the Rosetta space probe should use it to examine the surface of comet 67P/Churyumov-Gerasimenko.
History
Curium was discovered in the summer of 1944 by Glenn T. Seaborg and his collaborators Ralph A. James and Albert Ghiorso. In their series of experiments, they used a 60-inch cyclotron at the University of California at Berkeley. After neptunium and plutonium, it was the third transuranium discovered since 1940. It was produced before americium, which is one place lower in atomic number.
To create the new element, mostly the oxides of the starting elements were used. For this purpose, plutonium nitrate solution (containing the isotope 239Pu) was first applied to a platinum foil of about 0.5 cm2 , the solution was then evaporated and the residue was then annealed to the oxide (PuO2). After bombardment in the cyclotron, the coating was dissolved using nitric acid and then precipitated again with a concentrated aqueous ammonia solution as hydroxide; the residue was dissolved in perchloric acid. Further separation was carried out using ion exchangers. Two different isotopes were obtained in these series of experiments: 242Cm and 240Cm.
They produced the first isotope 242Cm in July/August 1944 by bombarding 239Pu with α-particles. This produces the desired isotope and a neutron in a so-called (α,n)-reaction:
The identification succeeded beyond doubt on the basis of the characteristic energy of the α-particle emitted during the decay. The half-life of this α-decay was determined for the first time to be 150 days (162.8 d).
They discovered the second, shorter-lived isotope 240Cm, also produced by bombarding 239Pu with α-particles, later in March 1945:
The half-life of the subsequent α-decay was determined for the first time to be 26.7 days (27 d).
Due to the ongoing Second World War, the discovery of the new element was not made public at first. The public only learned of its existence in an extremely curious way: on the American radio program Quiz Kids on November 11, 1945, one of the young listeners asked Glenn Seaborg, who appeared as a guest on the program, whether new elements had been discovered during World War II in the course of research into nuclear weapons. Seaborg answered in the affirmative, revealing the existence of the element simultaneously with that of the next lowest element, americium. This was before the official announcement at a symposium of the American Chemical Society.
The discovery of curium (242Cm, 240Cm), its production and that of its compounds were later patented under the name Element 96 and compositions thereof, with only Glenn T. Seaborg listed as the inventor.
The name curium was chosen by analogy with gadolinium, the rare earth metal that ranks just above curium in the periodic table. The choice of name honored the married couple Marie and Pierre Curie, whose scientific work had been groundbreaking in the study of radioactivity. It thus followed the naming of gadolinium, which was named after the famous researcher of rare earths, Johan Gadolin: As the name for the element of atomic number 96 we should like to propose "curium", with symbol Cm. The evidence indicates that element 96 contains seven 5f electrons and is thus analogous to the element gadolinium with its seven 4f electrons in the regular rare earth series. On this basis element 96 is named after the Curies in a manner analogous to the naming of gadolinium, in which the chemist Gadolin was honored.
· .jpg)
Marie Curie
· 
Pierre Curie
The first weighable amount of curium could be produced in 1947 in the form of the hydroxide by Louis B. Werner and Isadore Perlman. This was 40 μg of 242Cm produced by neutron bombardment of 241Am. It was not prepared in elemental form until 1951 by reduction of curium(III) fluoride with barium.
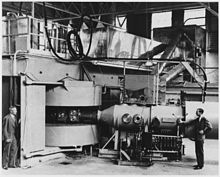
60 inch cyclotron

Glenn T. Seaborg
Occurrence
The longest-lived isotope 247Cm has a half-life of 15.6 million years. For this reason, all the primordial curium that the Earth contained at its formation has now decayed. Curium is produced artificially in small quantities for research purposes. It is also found in small quantities in spent nuclear fuel.
Most of the curium found in the environment comes from atmospheric nuclear weapons tests up to 1980. Locally, there are higher occurrences due to nuclear accidents and other nuclear weapons tests. However, curium makes little contribution to the Earth's natural background.
Beyond the initial discovery of einsteinium and fermium in the remains of the first American hydrogen bomb, Ivy Mike, on November 1, 1952, on Eniwetok Atoll, isotopes of curium, berkelium, and californium were found in addition to plutonium and americium: primarily the isotopes 245Cm and 246Cm, in smaller quantities 247Cm and 248Cm, and in trace amounts 249Cm. For reasons of military secrecy, the results were not published until later in 1956.
Search within the encyclopedia


