COVID-19 vaccine
![]()
This article describes vaccines that are newly developed and whose mode of action is under continuous investigation. The information may therefore change rapidly.
A SARS-CoV-2 vaccine (also referred to as COVID-19 vaccine, SARS-Coronavirus-2 vaccine or corona vaccine) is a vaccine (vaccine) against the novel coronavirus SARS-CoV-2. The aim of vaccine development is to generate an adaptive immune response in the vaccinated organism through vaccination, which protects against infection with the virus and thus against the COVID-19 disease. A distinction is made between active vaccines, which trigger a longer-term immune response against the virus in the vaccinated person after one to two weeks, and passive vaccines, which immunise immediately and directly against COVID-19, but only protect for a few weeks.
Like all medicines, COVID-19 vaccines undergo clinical trials before they can be submitted for marketing authorisation - on a country-by-country or cross-country basis - to the relevant regulatory authority. Although this process was faster than usual for the Corona vaccines, no testing step was omitted, shortened or simplified (in Europe). Instead, the speed was due in particular to new, improved technology, pre-existing knowledge from SARS-CoV-1, substantial financial support and the parallel conduct of the test phases (see also rolling review process). If significant efficacy or immunogenicity has been demonstrated and the benefit outweighs any potential risk from possible severe side effects, a vaccine is approved.
Worldwide, according to the World Health Organization (as of June 1, 2021), 102 vaccines are in clinical trials, 17 of which are in final Phase III trials. Another 185 are in preclinical development. In Russia, the vector vaccine Gam-COVID-Vac ("Sputnik V") was already approved in August 2020, but without waiting for the phase III trials with tens of thousands of subjects. In addition, the RNA vaccines tozinameran (Biontech/Pfizer) and mRNA-1273 (Moderna/NIAID), as well as the vector vaccines AZD1222 (AstraZeneca/University of Oxford) and Ad26.COV2.S (Janssen/Johnson & Johnson), among others, were approved based on the results of Phase 3 trials. They, as well as the vaccine BBIBP-CorV, were also included by the WHO in the list of vaccines for emergency use ("WHO emergency approval"). These WHO emergency approvals are used in countries without their own drug trials. Other vaccines are under review.
Initial studies from Israel and the UK suggest that third party infection is reduced by vaccination with tozinameran (Biontech) or AZD1222 (AstraZeneca).
For passive immunization against SARS-CoV-2, several emergency approvals for antibody preparations have been granted in the USA.
Following reports of a specific form of very rare cerebral venous thrombosis observed after vaccination with AZD1222 (AstraZeneca), several European countries suspended vaccination of this vaccine in mid-March 2021. On 18 March 2021, the European Medicines Agency (EMA) announced that the benefits of the vaccine were far superior to the potential risks.
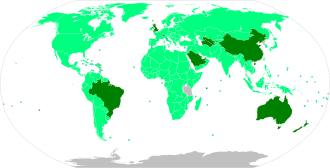
Map with approval status (as of June 2021) Approved for general use, mass vaccination ongoing Limited approval granted, mass vaccination underway Limited approval granted, limited vaccination Approved for general use, mass vaccination planned Limited approval granted, mass vaccination planned Approval pending → see below for details Approved vaccines
Immunology
Central antigens of SARS-CoV-2 (i.e. the "docking sites" for the antibodies) in vaccine development are two proteins of the viral envelope, the S-glycoprotein (spike glycoprotein, the viral docking protein on the surface of SARS-CoV-2) and the membrane protein (M) as well as the nucleocapsid protein in the virus interior.
The virus belongs to the order Nidovirales, family Coronaviridae, subfamily Orthocoronavirinae and subgenus Sarbecoviruses (as well as SARS-CoV). It is thus an enveloped, non-segmented RNA virus. Neutralizing antibodies have been described against two proteins of the viral envelope (S-glycoprotein and membrane protein) of SARS-CoV. Neutralizing antibodies against the S-glycoprotein are mainly responsible for protection against infection by SARS or MERS-CoV, but the cause of protection is probably dependent on the type of vaccine, antigens used, animal models, and mode of application. Conserved epitopes have been identified in the S-glycoprotein and nucleocapsid protein, which may be suitable for broad-acting vaccines. There is cross-reactivity in mice of neutralizing antibodies to the S-glycoprotein that inhibit cell entry of both SARS-CoV and SARS-CoV-2. Both SARS-associated viruses use the same receptor for cell entry, angiotensin-converting enzyme 2 (ACE2), whereas MERS-CoV uses dipeptidyl peptidase 4 (CD26). Numerous ACE-2 receptors in humans are also found in the intestinal tract, vascular cells, cardiac muscle, and kidney. The S-glycoprotein is divided into two subunits, S1 and S2. S1 contains the receptor binding domain and conditions binding to the host cell. S2 is responsible for fusion with the cell membrane. The binding affinity of SARS-CoV-2 to the ACE-2 receptor is approximately 10 to 20 times stronger than that of SARS-CoV. There were no monoclonal antibodies against the receptor-binding protein domain (RBD) of the S-glycoprotein of SARS-CoV in March 2020 that exhibited appreciable binding affinity to SARS-CoV-2. In the S-glycoprotein of SARS-CoV-2, 13 epitopes for MHC I (generate a cellular immune response) and 3 for MHC II (generate a humoral immune response) were identified.
A problem in vaccine development is the high mutation rate of some RNA viruses, which means that, as with influenza vaccine, the vaccine must be continuously adapted to the changing circulating virus strains or only cover part of the circulating virus strains. The receptor-binding protein domain of the S-glycoprotein (used as an antigen to generate neutralizing antibodies) is the most variable part of SARS-CoV-2, with strain D614G being the dominant global SARS-CoV-2 strain at approximately 85% as of November 2020. Almost all strains with this D614G mutation also show mutations in replication proteins such as ORF1ab P4715L and RdRp P323L. These in turn are the targets for some drugs such as remdesivir and favipiravir.
Another problem is that infection-enhancing antibodies (against proteins in the viral envelope) have been described in SARS-CoV and MERS-CoV, which are undesirable and may be suspected in SARS-CoV-2. To avoid infection-enhancing antibodies against the S-glycoprotein, immunization can presumably be done with truncated variants, such as the RBD or the S1 subunit of the S-glycoprotein. A third problem is that pulmonary alveoli immunopathogenesis by immigration of eosinophils and type 2 T helper cells was observed in a vaccine against SARS-CoV, which may be suspected in SARS-CoV-2 vaccines. Immunopathogenesis could be avoided in a SARS-CoV vaccine by the addition of a specific adjuvant (a delta-inulin-based polysaccharide). Therefore, criteria for SARS-CoV-2 vaccine development include: minimization of adverse immune reactions, suitability for vaccination of adult health care workers, suitability for vaccination of people with risk factors (people over 60 years of age or with diabetes mellitus or hypertension), and suitability for stockpiling as discussed in the COVID-19 vaccine prioritization.
Most SARS-CoV-2 vaccines use the S-glycoprotein of SARS-CoV-2. This peplomer is a fusogenic protein in function, allowing the virus to exit the endosome after uptake into a cell. As a fusogenic protein, it can adopt at least two protein folds: before fusion with the endosomal membrane and after fusion with the endosomal membrane. Some SARS-CoV-2 vaccines use as antigen a variant of the S-glycoprotein that has two modified prolines near the fusion domain that stabilise the protein fold before membrane fusion (2P-prefusion-stabilised). In the 2P variant, two amino acids were exchanged for prolines: at position 1060 there was previously a valine, and at position 1061 there was previously a leucine. The 2P variant was first described for coronaviruses in MERS-CoV. The analogy of the 2P variant in SARS-CoV-2 was confirmed.
Model of a SARS-CoV-2 virion with red coloured spikes
Vaccine Development
Predevelopment
Vaccines are the most effective preventive measures against infectious diseases. Research into vaccines against coronaviruses, including HCoV-HKU1, HCoV-NL63, HCoV-OC43, HCoV-229E, SARS-CoV and MERS-CoV, has therefore been ongoing for many years. There are several available animal vaccines against coronaviruses, such as avian coronavirus (in birds), canine coronavirus (in dogs), and feline coronavirus (in cats). For the human pathogenic coronaviruses SARS-CoV and MERS-CoV, experimental vaccines exist that have been tested in animals. Against SARS-CoV and against MERS-CoV, a total of four vaccines have been studied in humans with completed clinical trials through 2019. All four vaccines were safe and immunogenic. Six other vaccines were in clinical trials in 2019. However, none has a drug approval for humans yet. For MERS-CoV, reasons for this include the lack of cost-effective animal models, the sporadic and local occurrence of the virus, and the resulting lack of investment. In the case of SARS-CoV, no new infections occurred after 2004. Only with the COVID-19 pandemic from 2020 onwards did coronavirus vaccines become urgent again. Thanks to the above-mentioned research that had already been carried out, it was possible to build on existing knowledge and thus quickly develop a vaccine against SARS-CoV-2 as well. In this context, the new technology of RNA vaccines, which consist of a messenger RNA (mRNA) coding for one or more viral proteins, was also used. This protects the vaccinated person's immune system from the natural pathogen in the event of an actual infection. Their development and production can be much faster than with conventional vaccines. Previously, tests of RNA vaccines against other diseases had already been taking place in human clinical trials for several years.
Specific vaccine development for SARS-CoV-2
As of late January 2020, the Chinese Center for Disease Control and Prevention, the University of Hong Kong (nasally applied), Shanghai East Hospital, and several other universities including Washington University in St. Louis, among others, began vaccine development. Six vaccine developers were supported by the Coalition for Epidemic Preparedness Innovations (CEPI) in March 2020, including Curevac, Moderna (with the National Institute of Allergy and Infectious Diseases), Inovio Pharmaceuticals (with the Wistar Institute and Beijing Advaccine Biotechnology), the University of Queensland (with adjuvant manufacturer Dynavax), the University of Oxford, and Novavax. In early March 2020, CEPI announced $2 billion in funding for the development of SARS-CoV-2 vaccines through various public and private organizations, including participation from Germany, Denmark, Finland, the United Kingdom, and Norway.
Science magazine declared the development of vaccines against SARS-CoV-2 at an unprecedented rate as Breakthrough of the Year, the scientific breakthrough of the year.
.jpg)
Research on a vaccine in Japan
Search within the encyclopedia