Communes of the Haute-Corse department
The department of Haute-Corse is located in the region of Corsica in France. It is divided into three arrondissements with 236 communes (as of 1 January 2017).
See also: List of cantons in the department of Haute-Corse
Communes by department in France
Ain | Aisne | Allier | Alpes-de-Haute-Provence | Hautes-Alpes | Alpes-Maritimes | Ardèche | Ardennes | Ariège | Aube | Aude | Aveyron | Bouches-du-Rhône | Calvados | Cantal | Charente | Charente-Maritime | Cher | Corrèze | Corse-du-Sud | Haute-Corse | Côte-d'Or | Côtes-d'Armor | Creuse | Dordogne | Doubs | Drôme | Eure | Eure-et-Loir | Finistère | Gard | Haute-Garonne | Gers | Gironde | Hérault | Ille-et-Vilaine | Indre | Indre-et-Loire | Isère | Jura | Landes | Loir-et-Cher | Loire | Haute-Loire | Loire-Atlantique | Loiret | Lot | Lot-et-Garonne | Lozère | Maine-et-Loire | Manche | Marne | Haute-Marne | Mayenne | Meurthe-et-Moselle | Meuse | Morbihan | Moselle | Nièvre | Nord | Oise | Orne | Pas-de-Calais | Puy-de-Dôme | Pyrénées-Atlantiques | Hautes-Pyrénées | Pyrénées-Orientales | Bas-Rhin | Haut-Rhin | Rhône | Métropole de Lyon | Haute-Saône | Saône-et-Loire | Sarthe | Savoie | Haute-Savoie | Paris | Seine-Maritime | Seine-et-Marne | Yvelines | Deux-Sèvres | Somme | Tarn | Tarn-et-Garonne | Var | Vaucluse | Vendée | Vienne | Haute-Vienne | Vosges | Yonne | Territoire de Belfort | Essonne | Hauts-de-Seine | Seine-Saint-Denis | Val-de-Marne | Val-d'OiseGuadeloupe |
Martinique | French Guiana | Réunion | Mayotte | Saint-Pierre and Miquelon | French Polynesia | New Caledonia
[
{{7053}}]
In the quantum mechanical models of atoms, an atomic orbital is the spatial wave function of a single electron in a quantum mechanical state, usually in a steady state. Its formula symbol is usually φ 



In the older atomic models according to Niels Bohr (Bohr atomic model, 1913) and Arnold Sommerfeld (Bohr-Sommerfeld atomic model, 1916), an orbital describes an exact electron path selected by the quantisation rules. This idea was abandoned in quantum mechanics in favour of a diffuse distribution of the electron's residence probability. The quantum mechanical atomic orbital extends for bound electrons from the atomic nucleus in the centre outwards to infinity, where the residence probability asymptotically approaches zero. The most probable distance from the atomic nucleus for the innermost orbital is equal to the radius of the 1st Bohr circular orbit.
An orbital is usually represented by the surface of the smallest possible volume in whose interior the electron is located with a high (e.g. 90%) probability (see figure). One thus obtains bodies that correspond approximately to the size and shape of the atoms as they are noticeable in chemical molecules, condensed matter and the kinetic theory of gases.
The most common atomic orbitals are those that arise for the single electron of the hydrogen atom as solutions of the Schrödinger equation of the hydrogen problem and were first published in 1926. They have different forms 


In the orbital model for atoms with several electrons, it is assumed that the electrons distribute themselves among the orbitals taking into account the Pauli principle. Such a state is called the electron configuration and is often a useful approximation for the structure of the atomic shell, although this is further complicated by additional electron correlations.
To describe electrons in molecules, molecular orbitals are formed as linear combinations of atomic orbitals. Electrons in solids are described by orbitals that have the form of Bloch wave functions.
This article only deals with bound electrons in atoms. A simplification of the orbital model is the shell model.
Representation
Since the complete graphical representation of a wave function 





These proportions can be drawn separately. However, images of orbitals often show a representation of the probability density 

are defined. By sampling different angles θ 

Not infrequently, when representing an isosurface of 

A simple way of schematically representing the occupation of atomic orbitals is the Pauling notation.
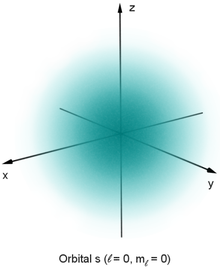
Representation of the probability density of the 1s orbital with the aid of a (very fine) point cloud
Quantum theory
→ Main article: Hydrogen problem
From the non-relativistic quantum theory, the orbitals result as follows: The interaction between electron and atomic nucleus is described by the Coulomb potential, the atomic nucleus is assumed to be fixed. The Hamilton operator for the one-electron system is
With the potential

Since the Hamiltonian operator commutes with the angular momentum operator, 


The Schrödinger equation
can be decomposed into a radius-dependent and an angle-dependent part. The eigenfunctions ψ 

These are shown normalised up to 


The orbitals shown in the following table are all aligned about the z-axis because they are eigenfunctions of the 



| Complex wave functions in hydrogen-like atoms | |||||
| Orbital | Wave function of the orbital | Shape of the orbital ψ | |||
| | | | | ||
| 1s | 1 | 0 | 00 | |
|
| 2s | 2 | 0 | 00 | |
|
| 2p0 | 2 | 1 | 00 | |
|
| 2p-1/+1 | 2 | 1 | ±1 | |
|
| 3s | 3 | 0 | 00 | |
|
| 3p0 | 3 | 1 | 00 | |
|
| 3p-1/+1 | 3 | 1 | ±1 | |
|
| 3d0 | 3 | 2 | 00 | |
|
| 3d-1/+1 | 3 | 2 | ±1 | |
|
| 3d-2/+2 | 3 | 2 | ±2 | |
|
Natural orbital
A natural orbital is an orbital that does not result as an eigenfunction of a Hamiltonian operator, but as an eigenfunction of a single-electron density operator. This is obtained from a given many-particle state, which can also contain electron correlations, for example, and thus goes beyond the scope of a single-particle model. The electron configuration formed with the natural orbitals gives the best approximation to the initially given many-particle state that is possible with a single-particle model.
Time dependence
If orbitals are defined as eigenfunctions of an operator corresponding to an energy, then these orbitals are stationary within the framework of the chosen model. Examples of this are the Hartree-Fock orbitals as eigenfunctions of the Fock operator 
Hybridisation
Some symmetries of chemical bonds seem to contradict the characteristic shapes of the orbitals. These bonds can be understood by the formation of hybrid orbitals, which can form in the presence of electrons with different orbital angular momentum if they are energetically nearly equivalent (see above).
Multi-electron wave functions
The interpretation of orbitals as wave functions of one electron each is only possible unambiguously for single-electron systems. A wave function for N electrons can then be constructed by inserting N orbitals into a Slater determinant. This guarantees the antisymmetry of the entire wave function necessary for fermions, but cannot represent electron correlations beyond this. In order to also take the electron-electron interaction into account approximately, the orbitals can be determined by Hartree-Fock, Kohn-Sham calculations (see: Density Functional Theory in Quantum Physics) or MCSCF calculations (MCSCF: Multiconfiguration Self Consistent Field). However, it always remains valid that differently chosen orbitals, if they are linearly independent linear combinations of the original ones, mathematically result in the same Slater determinant, so that one cannot unambiguously infer from a given multi-particle wave function which are the individual occupied orbitals.
Representation of different orbitals of the first and second electron shell.
Top row: Representation of probability densities 
Bottom row: Representation of isosurfaces of 
Questions and Answers
Q: How many communes are there in the Haute-Corse department of France?
A: There are 236 communes in the Haute-Corse department of France.
Q: What are communes?
A: Communes in France are the smallest administrative division of the country.
Q: What is the Haute-Corse department?
A: The Haute-Corse department is a department in the northern part of the island of Corsica in France.
Q: What is the purpose of intercommunalities?
A: Intercommunalities in France are created to allow for cooperation between neighboring communes on issues of common interest.
Q: How many intercommunalities are there in Haute-Corse department?
A: The text does not specify the number of intercommunalities in the Haute-Corse department.
Q: What is the significance of the year 2018 mentioned in the text?
A: The year 2018 is mentioned as the latest year for which information about the intercommunalities is available.
Q: Can communes cooperate with each other outside of intercommunalities?
A: Yes, communes can cooperate with each other outside of intercommunalities as well.
Search within the encyclopedia


































