Chemical reaction
A chemical reaction is a process in which one or usually several chemical compounds are converted into others and energy is released or absorbed. Elements can also be involved in reactions. Chemical reactions usually involve changes in the chemical bonds in molecules or crystals. A chemical reaction can greatly change the properties of the products compared to the reactants. Chemical reactions do not include physical processes in which only the state of aggregation changes, such as melting or evaporation, diffusion, the mixing of pure substances to form mixtures of substances, and nuclear reactions in which elements are converted into others.
Reactions consist of a usually quite complicated sequence of individual partial steps, the so-called elementary reactions, which together form the overall reaction. Information about the exact sequence of the partial steps is provided by the reaction mechanism. The reaction equation is used to describe chemical reactions, in which reactants, products and sometimes also important intermediates are represented graphically and are connected to each other by an arrow, the reaction arrow.
Both elementary reactions and reaction mechanisms can be divided into different groups. Elementary reactions include, for example, the decay of one molecule into two or the reverse case, the synthesis of two atoms or molecules into one. Reaction mechanisms are often classified according to the change that occurs in the substances involved. If, for example, there is a change in the oxidation numbers, one speaks of oxidation and reduction; if a solid product is formed from dissolved substances, one speaks of precipitation.
The extent to which a certain reaction of two or more partners takes place depends on the difference between the Gibbs energy of the products and the reactants, which is composed of an enthalpic and an entropic part. For negative values, the reaction equilibrium is on the side of the products. However, there are also many reactions that are thermodynamically possible in this sense, but kinetically proceed only very slowly, in extreme cases so slowly that they can practically not be observed. The reason for this is that the activation energy that has to be applied is too high for the further reaction to be possible. However, such reactions run faster at higher temperatures, since a comparatively larger number of the particles involved have enough energy to overcome the activation barrier. In many reactions this is also possible by means of catalysis, in which not the direct reaction takes place, but another one, in which a third substance, unchanged from the reaction, is involved. The presence of this catalyst lowers the required activation energy.

Thermite reaction
History
Chemical reactions such as combustion in a fire, alcoholic fermentation or the reduction of ores to metals - in the case of iron, for example - have been known for a very long time. The first theories on the transformation of substances were developed by Greek philosophers, such as the four-element theory of Empedocles, according to which every substance is composed of the four basic elements fire, water, air and earth and can also be broken down into these. In the Middle Ages, it was mainly the alchemists who were concerned with chemical reactions. In particular, they tried to convert lead into gold, using, among other things, reactions of lead and lead-copper alloys with sulfur.
The production of chemical substances that do not occur in nature through suitable reactions has been known for a long time. This applies, for example, to sulphuric and nitric acid, the first production of which is attributed to the controversial alchemist Jābir ibn Hayyān. They were produced by heating sulphate and nitrate ores such as copper vitriol, alum and saltpetre. In the 17th century, Johann Rudolph Glauber first produced hydrochloric acid and sodium sulfate by reacting sulfuric acid and sodium chloride. With the development of the lead chamber process for sulfuric acid production and the Leblanc process for sodium carbonate production, chemical reactions were also used industrially. With increasing industrialization, industrial synthesis became more important and newer and more efficient processes were developed. Examples include the contact process used from 1870 onwards for sulphuric acid production and the Haber-Bosch process developed in 1910 for ammonia synthesis.
From the 16th century onwards, researchers such as Johan Baptista van Helmont, Robert Boyle or Isaac Newton attempted to scientifically investigate observed chemical transformations and to establish theories on their course. One important reaction studied was combustion, for which Johann Joachim Becher and Georg Ernst Stahl developed the phlogiston theory at the beginning of the 18th century. However, this proved to be incorrect and was refuted in 1785 by Antoine Lavoisier, who found the correct explanation of combustion as a reaction with oxygen in the air.
In 1808, Joseph Louis Gay-Lussac recognized that gases always react with each other in certain ratios. From this and from Dalton's atomic theory, Joseph Louis Proust developed the law of constant proportions, on which stoichiometry is based and which also enabled the development of the reaction equations.
For organic reactions, it was long assumed that they were determined by a special "vital force" (vis vitalis) and thus differed from non-organic reactions. After the synthesis of urea from inorganic precursors by Friedrich Wöhler in 1828, this assumption lost much of its significance in chemistry. Other chemists who made important contributions to the elucidation of organic chemical reactions were, for example, Justus von Liebig with his radical theory, Alexander William Williamson, who developed the synthesis of ethers named after him, and Christopher Kelk Ingold, who, among other things, explored the mechanisms for substitution reactions.
.jpg)
Antoine Lavoisier developed the theory of combustion as a chemical reaction with oxygen
Reaction Equations
→ Main article: Reaction equation
To represent chemical reactions graphically, so-called reaction equations are used. These consist of the sum or structural formulae of the reactants on the left and those of the products on the right. Between them is an arrow, the so-called reaction arrow, which indicates the direction and type of reaction. The tip of the arrow always points in the direction of the reaction. For equilibrium reactions, double arrows pointing in opposite directions are used. Reaction equations should be stoichiometrically balanced. This means that there should be the same number of atoms on both sides of the reaction arrow and equations should be balanced by different numbers of molecules involved, if necessary.
Schematic simple reaction equation
More complicated reactions are represented by formula diagrams that show not only reactants and products but also important intermediates or transition states. Here, the reaction paths are clarified by arrows showing the attack of electron pairs of one atom on other atoms. In reaction equations of organic chemistry, small, inorganic molecules such as water or carbon dioxide, are often placed on the arrow (for reactants) or below (for products) or indicated by signs. Catalysts, solvents, special conditions or other substances that play a role during the reaction but do not change during it are also written on the reaction arrow.
For the planning of complicated syntheses, the notation of a reaction as retrosynthesis can also be useful. Here, a reaction is written down from the end, i.e. the product, which is decomposed via possible synthesis steps until possible reactants are reached. Retrosyntheses are indicated by a special arrow, the retrosynthesis arrow ( 
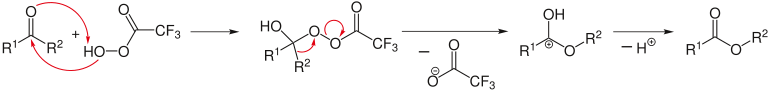
Typical reaction mechanism of organic chemistry (example: Baeyer-Villiger oxidation based on the reaction of a percarboxylic acid with a ketone)
Questions and Answers
Q: What is a chemical reaction?
A: A chemical reaction happens when one or more chemicals are changed into one or more other chemicals.
Q: Can you give examples of chemical reactions?
A: Yes, some examples of chemical reactions are iron and oxygen combining to make rust, vinegar and baking soda combining to make sodium acetate, carbon dioxide and water, things burning or exploding, and many reactions that happen inside living things, such as photosynthesis.
Q: Are all chemical reactions fast?
A: No, some reactions are fast, and others are slow. Some happen at different speeds, depending on temperature or other things.
Q: What is an exothermic reaction?
A: An exothermic reaction is a reaction that gives out energy.
Q: What is an endothermic reaction?
A: An endothermic reaction is a reaction that takes in energy.
Q: Are nuclear reactions considered chemical reactions?
A: No, nuclear reactions are not chemical reactions. Chemical reactions involve only the electrons of atoms; nuclear reactions involve the protons and neutrons in the atomic nuclei.
Q: Can temperature affect the speed of a chemical reaction?
A: Yes, depending on the temperature or other things, some reactions can happen at different speeds. For example, wood does not react with air when it is cold, but if it is made hot enough, it will start to burn.
Search within the encyclopedia
