Chemical bond
Chemical bonding is a physico-chemical phenomenon by which two or more atoms or ions are tightly bound together to form chemical compounds. This is based on the fact that it is energetically more favourable for most atoms or ions to be bound to suitable bonding partners instead of being present as a single (unbound) particle.
The basis of bonding is electrostatic interactions or interactions of the electrons of two or more atoms. In many cases, both bonding mechanisms play a role. Parameters that are important for describing a bond and can be studied experimentally are the bond length as a measure of the distance between two atomic nuclei and the bond energy, which indicates the strength of a bond. The chemical bond is the basis for molecules and thus for chemical compounds to form at all, and is thus one of the most important foundations of chemistry.
Chemical bonds can be divided into different types. In ionic crystals, the ionic bond based on electrostatic interactions predominates, in metals the metallic bond based on freely moving electrons. In contrast, the formation of molecules and complexes is based on localized bonds, which are based on the formation of electron pairs. Within localized electron pair bonds, a distinction is often made between covalent bonding, in which each atom contributes an electron to the bond, and coordinative bonding in complexes, in which a pair of electrons from a ligand interacts with an empty orbital of the central atom. In special cases, multicenter bonds can occur. Metallic, ionic and covalent bonds are idealizations of chemical bonds.
Sometimes weak interactions, such as Van der Waals interactions, dipole interactions and hydrogen bonding are counted as chemical bonds. However, these are not fixed chemical bonds, but weak attractive forces that act between individual molecules.
For the description of the bonds in molecules, various theories have been established in theoretical chemistry, which, however, are all only approximations of the actual bonding situation that are as exact as possible. These include the valence structure theory and the molecular orbital theory.
Bonds can be broken by the application of energy, for example in the form of heat or light. The resulting single atoms or molecules often have a high tendency to rebond. The rebonding may occur at the previously cleaved site, or it may occur at other atoms or molecules. This is a basis for chemical reactions.
History
The development of various theories of chemical bonding is closely linked to the development of theories and experiments on the shape of the single atom. The first concrete theories were put forward after the discovery of the electron by Joseph John Thomson in 1897. In his model of the atom, Thomson imagined that chemical bonds were based on electrostatic forces created by the transfer from one atom to another. This initially led to the assumption that chemical bonds must always be polar in structure.
Based on the properties of organic compounds that could not be explained by polar bonds and experiments with channel beams, it soon became clear that there must also be a nonpolar bond. Gilbert Lewis first suggested in 1916 that the nonpolar bond was due to paired electrons. This theory was also compatible with the atomic models of Rutherford and Bohr, which had meanwhile replaced Thomson's model.
With the development of quantum mechanics and especially the establishment of the Schrödinger equation by Erwin Schrödinger in 1926, more precise theories of binding could be established. The first quantum mechanical theory was developed with the valence structure theory in 1927 by Walter Heitler and Fritz London. The original theory was initially valid only for the simplest molecule, the H2+ ion of two protons and one electron. Linus Pauling extended the theory extensively by introducing the orbital and hybridization, so that the theory could be applied to more complicated molecules.
Also in 1927, the more precise molecular orbital theory was established by Friedrich Hund and Robert Mulliken. This too was initially only applicable to simple molecules, but was gradually extended, for example in 1930 by Erich Hückel by a more precise explanation of multiple bonds with the explanation of the π-bond.
After the basic quantum mechanical theories were established, various researchers attempted to use these theories to explain observed phenomena in organic or inorganic chemistry. Important examples are the ligand field theory for complexes, published in 1951 by Hermann Hartmann and F. E. Ilse, and the Woodward-Hoffmann rules established in 1968 by Robert B. Woodward and Roald Hoffmann, with which a certain type of organic reactions, the pericyclic reactions, could be understood on the basis of molecular orbital theory.
With the development of the computer from about 1950 onwards, more complicated theoretical calculations on chemical bonding also became possible. An important development for this were, among others, those of the Roothaan-Hall equations by Clemens C. J. Roothaan and George G. Hall in 1951, which are important in the Hartree-Fock method. Finally, starting in 1964, another way to theoretically calculate chemical bonding was developed by Walter Kohn in the form of density functional theory. He received the Nobel Prize in Chemistry for this in 1998.

Linus Pauling received the Nobel Prize in Chemistry in 1954 for his work on chemical bonding, among other things.
Ionic bond
→ Main article: Ionic bond
The ionic bond is an undirected bond with a large range that acts equally strongly in all spatial directions. It is the predominant type of bond in salts, i.e. compounds of metals and nonmetals that are periodically arranged in lattices. During the reaction of metals and nonmetals, the large electronegativity difference results in the transfer of valence electrons of the metal to the nonmetal and thus to electrically charged atoms, the so-called ions. The larger the electronegativity difference, the more valence electrons are transferred and the more ionic the bond. However, in all ionic bonds there are also covalent parts to the bond. If the differences are weak, only a small amount of transfer occurs and it is necessary to consider both parts to describe the bond.
For the bonding in ion crystals, electrostatic interactions between the differently charged ions are mainly responsible. The energetic structure can be described theoretically well with the lattice energy. For this purpose, mainly the attractive and repulsive forces between the ions, as well as the repulsion of the interpenetrating electron shells are included and Coulomb's law is taken into account. The type of lattice is also included via the Madelung constant.
The ionic bond is a strong bond. Typical values for lattice energies of ionic substances are 787 kJ/mol (8.2 eV) for sodium chloride and 3850 kJ/mol (39.9 eV) for the more highly charged magnesium oxide (determined via the Born-Haber cycle). This accounts for the high melting temperatures of many ionically structured substances. However, because the bond is undirected, it is no stronger than many covalent bonds, which act only within a molecule and not between molecules of a substance. The electrostatic nature of ionic bonding causes the brittleness of many ionic crystals, as displacements between ions easily cause like-charged ions to adjoin and repel each other, thus blowing the crystal apart.
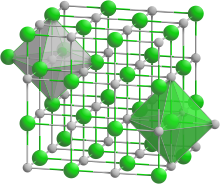
Stylized ion lattice (sodium chloride structure)
Questions and Answers
Q: What is a chemical bond?
A: A chemical bond is a type of attraction force that holds together different chemical species.
Q: What happens to atoms that are bonded together?
A: Atoms that are bonded together stay together unless the needed amount of energy is transferred to the bond.
Q: What comes with strong chemical bonding?
A: Strong chemical bonding comes with the sharing or transfer of electrons between the participating atoms.
Q: What are the types of chemical bonds?
A: The types of chemical bonds are covalent and ionic.
Q: How are covalent bonds formed?
A: Covalent bonds are formed when atoms share electrons.
Q: What is ionic bonding?
A: Ionic bonding is the attraction between oppositely charged ions.
Q: How do chemists typically describe chemical bonds?
A: Chemists typically describe chemical bonds through the number of electrons each atom has on itself, drawing them as dots or lines to form a maximum of eight, and drawing a line between the two electrons if they form a chemical bond.
Search within the encyclopedia