Cardiomyopathy
Cardiomyopathies (syn. myocardiopathies, gr. kardía (καρδία) heart, gr. mys (μυς) muscle, gr. páthos (πάθος) ailment) are a heterogeneous group of diseases of the heart muscle that are associated with mechanical and/or electrical dysfunction and usually, but not necessarily, cause inappropriate hypertrophy (thickening) or dilatation (widening) of one or both ventricles. Their causes are varied and often genetic. Cardiomyopathies are either limited to the heart or are part of a general systemic disease, often leading to cardiovascular-related death or progressive disability from heart failure.
"Cardiomyopathies are a heterogeneous group of diseases of the myocardium associated with mechanical and/or electrical dysfunction that usually (but not invariably) exhibit inappropriate ventricular hypertrophy or dilatation and are due to a variety of causes that frequently are genetic. Cardiomyopathies either are confined to the heart or are part of generalized systemic disorders, often leading to cardiovascular death or progressive heart failure-related disability".
- American Heart Association (AHA), 2006
To be distinguished are diseases that are a direct consequence of other cardiovascular anomalies, such as valvular heart disease, hypertension, congenital heart defects or the consequences of atherosclerotic coronary artery disease.
"It is also important to specify those disease entities that have not been included as cardiomyopathies in the present contemporary classification. These include pathological myocardial processes and dysfunction that are a direct consequence of other cardiovascular abnormalities such as that which occurs with valvular heart disease, systemic hypertension, congenital heart disease, and atherosclerotic coronary artery disease producing ischemic myocardial damage secondary to impairment in coronary flow."
- American Heart Association, 2006
Based on numerous new findings, this updated definition was proposed by the American Heart Association (AHA) in March 2006 and is now generally accepted. In addition to the above, a new classification distinguishes primary from secondary cardiomyopathies. Primary cardiomyopathies are in turn subdivided into congenital, acquired and mixed forms.
History
In the mid-1800s, chronic myocarditis alone was known as myocardial disease. Around 1900, the term primary myocardial disease was coined, and it was not until 1957 that the term cardiomyopathy emerged. There were several definitions until 1980, when the WHO referred to cardiomyopathy as "myocardial disease of unknown cause". The 1995 WHO classification expanded the term to "cardiomyopathies that result in dysfunction of the heart." New diseases such as arrhythmogenic right ventricular and restrictive cardiomyopathy were included.
Primary cardiomyopathies
Congenital primary cardiomyopathies
hypertrophic cardiomyopathy
→ Main article Hypertrophic cardiomyopathy
Hypertrophic cardiomyopathy (HCM) is characterized by a usually asymmetric thickening of the muscles of the left ventricle.
Arrhythmogenic right ventricular cardiomyopathy
Arrhythmogenic right ventricular cardiomyopathy (ARVCM), formerly also called arrhythmogenic right ventricular dysplasia (ARVD), is a predominantly congenital disease. In Veneto, the prevalence is particularly high, ranging from 1:2000 to 1:5000. However, there are also cases of ARVCM in Germany. As the disease progresses, more and more musculature of the right ventricle is replaced by fatty tissue, causing the right ventricle to enlarge. Rarely, restrictions in the pumping function of the heart are found. More common is sudden cardiac death (PHT) or "near" PHT triggered by physical exertion, such as competitive sports, especially in young people. Diagnosis can be made by echocardiography, MRI, ECG and McKenna score. A cardioverter defibrillator may be implanted for treatment. Athletic exertion should be avoided. Heart transplantation is the last resort in many advanced cases. Since ARVCM is inherited in many cases, it is advisable to have the family members of those affected tested. In some countries, for example Italy and the United States, all members of sports clubs are prophylactically screened.
One cause of arrhythmogenic right ventricular cardiomyopathy may be mutations in desmosome proteins. Desmosomes are important for cell contact of cells. In the case of ARVCM specifically for the myocardium. In Naxos disease, for example, the DSP gene is affected by a mutation that codes for the cell adhesion protein desmoplakin. The gene defect leads to arrhythmogenic right ventricular cardiomyopathy in affected patients. Mutations in the DES gene, which codes for the intermediate filament desmin, can also lead to ARVCM.
Left ventricular hypertrabeculation
Congenital, rare myocardial disease with spongy distended muscles, especially in the apex of the left ventricle, showing deep hollows (sinusoids) between muscle fibers (trabeculae) connected to the cardiac cavity. In isolated noncompaction of the myocardium (syn: noncompaction cardiomyopathy, left ventricular hypertrabeculation, spongy myocardium), the myocardium has not further compacted from its loose meshwork during the early embryonic period (spongy myocardium). Common in skeletal muscle diseases, also in combination with complex cyanotic heart defects.
The diagnosis is made by echocardiography, MRI or angiography of the left ventricle during cardiac catheterization. The clinical course is unclear. Cases of severe heart failure, thromboembolism, arrhythmias and sudden cardiac death have been reported. Familial clustered cases have been described, with mutations of the Z-disk, mitochondrial and G4.5 gene for tafazzin isolated.
Glycogen storage diseases
A distinction is made between PRKAG2 and Danon, a type II glycogenosis.
Line defects
Lenègre disease is a primary progressive conduction defect of the His-Purkinje system leading to widening of the QRS complex on the ECG with long pauses, bradycardia and syncope.
Sick sinus syndrome phenotypically resembles a conduction defect and may be autosomal dominant.
Wolff-Parkinson-White syndrome (WPW) also rarely occurs in families.
Mitochondrial myopathies
In part, the enzymes of the respiratory chain are encoded on the genes of the mitochondria. Several syndromes due to corresponding gene defects are known, including the
- Kearns-Sayre syndrome with pigmentary degeneration of the retina of the eye, ocular muscle paralysis and cardiomyopathy, and the
- MELAS syndrome with myopathy, encephalopathy, lactic acidosis and stroke-like episodes. In addition to the four features defined by the acronym, hypertrophic cardiomyopathy and diffuse coronary vascular disease are typical. Treatment is difficult. Some patients have been treated with coenzyme Q with modest short-term success. Heart transplantation is an alternative.
Ion channel defects
There is a growing list of rare hereditary and congenital cardiac arrhythmias encoded by genes for defective ion channel proteins. Also, a small proportion of 5-10% of children with suddeninfant death syndrome might be caused by ion channel defects. Clinical diagnosis of an ion channel defect is often possible phenotypically by a standard 12-lead ECG. Some of these cases have previously been classified as idiopathic ventricular fibrillation.
Long QT syndrome (LQTS)
Long QT syndrome is probably the most common of the ion channel diseases. Characteristic are prolongations of the ventricular depolarization and the QT interval in the 12-lead ECG. A special form of ventricular tachycardia (torsade de pointes) occurs and with it a risk of syncope and sudden cardiac death.
Jervell and Lange-Nielsen syndrome: rare, autosomal recessive, associated with deafness. Two genes encoding a slow activating delayed potassium channel.
Romano-Ward syndrome: Much more common, autosomal dominant, thirteen different genes, five of which (KCNQ1, KCNH2, KCNE1, KCNE2, KCNJ2) code for various potassium channels (I(K)), two (SCN5A, SCN3B) for cardiac sodium channels (I(Na)), and one (ANK2) for the protein ankyrin B, which is responsible for anchoring ion channels in the cell membrane, among others.
Brugada Syndrome
→ Main article: Brugada syndrome
As a clinical entity, Brugada syndrome has been known since 1992, it is sometimes called Brugada-Brugada syndrome after the two brothers who first described it, Pedro and Josep Brugada. It is sometimes responsible for sudden cardiac death, especially in young people. It is characterized by right bundle-branch block-like changes in the ECG, which may be provoked by an ajmaline test. Various genetic defects are known, they were described by Ramon Brugada, the youngest of the three Brugada brothers.
Asian SUNDS
SUNDS = "sudden unexplained nocturnal death syndrome". Predominantly in young Asian men, especially from Thailand, Japan, the Philippines and Cambodia. Sudden death during sleep due to ventricular tachycardia or ventricular fibrillation. Some cases are indistinguishable in appearance from Brugada syndrome. SUNDS made its way into the "1000 Ways to Bite the Dust" series as Death #818, entitled Fatal Dream.
Short QT Syndrome (SQTS)
First described in 2000. The QT interval in the ECG is shortened to less than 330 ms. There is a great risk of sudden cardiac death.
CPVT
CPVT = "Catecholaminergic Polymorphic Ventricular Tachycardia", first described by Coumel in 1978, characterized by a striking widening of the QRS complex in the ECG as well as by syncope, polymorphic ventricular tachycardia triggered by physical exertion or by violent emotional movements in children and adolescents, and by an increased risk of sudden cardiac death. CPVT is caused by mutations in the gene RYR2, which encodes the cardiac (=subtype 2) ryanodine receptor that is predominantly expressed in cardiac muscle. The inheritance of the mutation in the RYR2 gene within the affected families follows an autosomal dominant inheritance pattern, i.e. the risk for a first-degree relative to also inherit the disease-causing mutation is 50%. In 30% of diagnosed cases of CPVT, a previous sudden cardiac death is observed in family members.
Mixed (congenital and acquired) primary cardiomyopathies
dilated cardiomyopathy
Dilated cardiomyopathy (DCM), in which initially the left ventricle (heart chamber) (in the final stage also all heart cavities) is considerably dilated (the heart can be compared to a large flaccid sac). The wall thicknesses are usually not thickened or only slightly thickened (hypertrophied). The heart contracts only to a limited extent (= systolic functional restriction), often combined with asynchronous contraction of the chambers, due to a disturbance in conduction as a result of left bundle branch block. In terms of numbers, expired myocarditis and chronic alcohol abuse are the most frequent causes. There are also congenital forms. Secondary forms include "ischemic DCM" due to coronary artery disease and the end-state of a hypertensive heart. DCM is a common reason for heart transplantation when the patient's condition cannot be adequately improved with medications, coronary intervention, or cardiac resynchronization therapy (CRT). Diagnosis is confirmed by imaging (echocardiography, MRI, MSCT) and fine tissue (myocardial biopsy) after clinical suspicion with typical symptoms. Coronary artery disease must be ruled out by cardiac catheterization, as this could result in a curative treatment option for the cause.
Restrictive cardiomyopathy
Restrictive cardiomyopathy (RCM) presents with normal sized ventricles and mostly normal systolic pump function. Due to increased incorporation of connective tissue into the cardiac musculature, the cardiac musculature hardens. The resulting stiffening of the ventricles makes it difficult for the heart to fill during the slackening phase (diastole), and the blood backs up in the atria, which are greatly enlarged as a result. The wall thickness of the left ventricle is normal and the heart valves are regular.
Patients become conspicuous by symptoms of heart failure such as dyspnea and leg edema. The disease is extremely rare in industrialized countries and can be overlooked if it is not specifically looked for. The main cause is amyloidosis; sporadic and familial forms are known. For example, mutations in the DES gene, which encodes the intermediate filament protein desmin, have been identified. In tropical countries, however, restrictive cardiomyopathy is far more common, causing up to 20% of all cardiovascular deaths. Diagnostic methods include echocardiography, with tissue Doppler if necessary, cardiac catheterization with hemodynamic measurement, myocardial biopsy if necessary, and MRI.
Acquired primary cardiomyopathies
Myocarditis: inflammatory cardiomyopathy
Myocarditis is an acute or chronic process that may be caused by a wide range of
- toxins, like cocaine,
- endogenous substances, e.g. interleukin-2,
- infectious pathogens such as
- Viruses, e.g. coxsackie virus, adenovirus, parvovirus B19, human herpes virus 6 (HHV6), HIV, (the involvement of the hepatitis C virus is controversial)
- Bacteria, e.g. diphtheria, meningococcus, psittacosis, streptococcus, borrelia
- Rickettsia, e.g. spotted fever, Rocky Mountain spotted fever
- Fungi, e.g. Aspergillus, Candida
- parasites, e.g. Trypanosoma cruzi (Chagas disease), toxoplasmosis
- Whipple's disease (intestinal lipodystrophy)
- autoimmune (giant cell myocarditis) or in the context of a
- Hypersensitivity reactions e.g. to drugs such as antibiotics, sulphonamides, anticonvulsants and antirheumatic drugs.
Endocardial fibroelastosis in newborns and infants is the result of intrauterine infection with mumps virus.
Stress provoked (Tako-Tsubo)
Tako-Tsubo cardiomyopathy (syn: stress cardiomyopathy, broken heart syndrome, apical ballooning) is a myocardial disease that usually occurs in postmenopausal women and frequently after emotional stress situations and appears like an acute myocardial infarction in terms of symptoms, ECG changes and laboratory findings. There is a balloon-like distension of the apex of the ventricle as in a severe anterior wall infarction, but no narrowing or occlusion of the coronary vessels is found. The cause is thought to be a stress hormone-induced, only temporary occlusion of the fine hair vessels of the coronary arteries. This leads to a temporary "stunning" of the heart muscle, which, unlike in a real heart attack, does not die (necrosis) but can recover completely. The prognosis is usually good and in a few months the heart muscle disorder is regressive. The mortality rate is about 3%. Diagnosis is made by echocardiography, cardiac catheterization and magnetic resonance imaging (MRI).
Pregnancy Cardiomyopathy
Gestational or peripartum cardiomyopathy is a rare cause of acquired dilated cardiomyopathy with systolic heart failure in pregnant women in the last trimester or up to 5 months after delivery (peripartum cardiomyopathy). The cause is unclear, an inflammatory component (myocarditis), immune activating processes and gestational hypertension are discussed as triggers. Mostly overweight pregnant women over 30 years of age are affected, who have already given birth several times and have had pre-eclampsia. About half of the patients almost recover after six months, but in individual cases progressive heart failure with death or heart transplantation may occur.
Tachycardiomyopathy
Tachycardia-induced cardiomyopathy (synonym: tachymyopathy) is a potentially reversible impairment of predominantly left ventricular pump function that occurs in the setting of a prolonged tachycardic arrhythmia, usually rapid atrial fibrillation. The diagnosis is made by echocardiography together with the ECG. Therapeutically, the heart rate is first lowered with cardiac glycosides as well as with beta-blockers. In case of beta-blocker intolerance, calcium antagonists of the verapamil or diltiazem type can also be used. If the rate is not reduced sufficiently, dronedarone or amiodarone can be used. Heart failure therapy is then started. If there is no improvement after a short time and no extensive normalization of the heart's pumping capacity after several weeks of optimal therapy, other causes must be sought.
Newborns of diabetic mothers with poor metabolic status
This transient and rare form of non-familial cardiomyopathy is observed in children whose diabetic mothers had poor metabolic status (high blood glucose levels) during pregnancy. It often occurs together with fetal macrosomia.
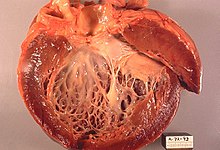
Dilated left ventricle cardiomyopathy. Opened heart
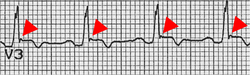
Epsilon wave (red arrow) as ECG indication of ARCM
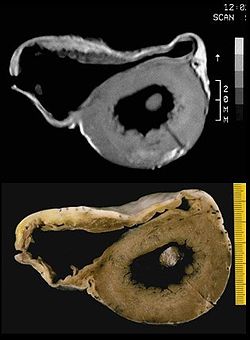
MRI image and pathological preparation of an ARVCM
Questions and Answers
Q: What is cardiomyopathy?
A: Cardiomyopathy is a heart muscle disease that impairs the function of the myocardium.
Q: What are the consequences of having cardiomyopathy?
A: People with cardiomyopathy are often at risk of arrhythmia and/or sudden cardiac death.
Q: How many types of cardiomyopathy are there?
A: There are four main types of cardiomyopathy.
Q: What is the reason behind cardiomyopathies?
A: The function of the myocardium is impaired for any reason in cardiomyopathies.
Q: Is there only one cause for cardiomyopathy?
A: No, there are multiple reasons for cardiomyopathy.
Q: Is cardiomyopathy a common illness?
A: Cardiomyopathy is not a common illness, but it is a dangerous one.
Q: Can people with cardiomyopathy lead a completely normal life?
A: It depends on the severity of the condition, but people with cardiomyopathy may need to take precautions and undergo treatment in order to lead a normal life.
Search within the encyclopedia