Boiling point
The boiling point (abbreviation: Sdp. ), evaporation point or boiling point (abbreviation: Kp. ) of a pure substance is a pair of values in its phase diagram and consists of two variables: the saturation temperature (especially also boiling temperature) and the saturation vapour pressure (especially also boiling pressure) at the phase boundary line between gas and liquid. It is therefore made up of the two state variables pressure and temperature during the transition of a substance from the liquid to the gaseous state of aggregation. For an open liquid, the boiling point is therefore the point on the temperature scale at which the vapour pressure is equal to the atmospheric pressure.
The boiling point represents the conditions that exist during the phase transition of a substance from the liquid to the gaseous phase, which is called boiling. In addition, it is identical to the condensation point for the reverse process of condensation, but only for pure substances. When a mixture of substances evaporates, a change in boiling behavior occurs, and a boiling range is observed instead of a single boiling point. In the case of a phase transition from the liquid to the gaseous phase below the boiling point, one speaks of evaporation.
In tables, the boiling temperatures are given at normal pressure, i.e. at 1013.25 hPa. This boiling point is referred to as the normal boiling point, and the specified boiling temperature as the normal boiling temperature (TSied). One method for estimating it is the Pailhes method, while the Guldberg rule establishes a relationship with the critical temperature. The term boiling point is often used as a short form for the normal boiling temperature and is therefore usually its synonym in common usage, which would, however, reduce the boiling point to only one pair of values and is therefore formally incorrect.
In a pressure cooker, for example, one makes use of the fact that the boiling temperature and the boiling pressure depend on each other. By increasing the pressure, usually by one bar (1000 hPa), the boiling temperature of the water is increased from 100 °C to about 120 °C. Both temperatures are boiling temperatures, but only the value of 100 °C is also the boiling temperature. Both temperatures represent boiling temperatures, but only the value of 100 °C is also the boiling temperature under normal pressure and thus the normal boiling temperature. Mixing the two terms is therefore unspecific, by no means self-evident and should be avoided.
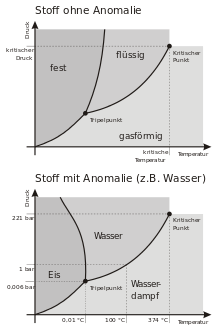
Phase diagram of an "ordinary" substance and water
Boiling process
→ Main article: Evaporation, heat of evaporation and boiling delay
Below and above the boiling point, heating the liquid or gas only leads to an increase in temperature. The energy supplied is converted into kinetic energy of the particles. During the phase transition of the liquid to the gas, however, the temperature remains constant, provided that the pressure also remains constant. All thermal energy supplied is invested in the change of state.
Once the boiling point is reached, the chemical-physical interactions between the particles are dissolved when further energy is added - the particles enter the gas phase. The temperature of the liquid stagnates, since the thermal energy supplied is used entirely for the dissolution of the intermolecular bonds. The energy required for this in the case of one mole of the substance is also referred to as the enthalpy of vaporization and its counterpart, which is not related to the quantity of substance, as the heat of vaporization. Only when all particles are in the gas phase does the temperature of the system rise again.
Water, hydrogen peroxide or alkalis (for example caustic soda) without dust particles or gas bubbles can also be heated above boiling temperature in clean vessels without boiling occurring. The smallest disturbances, such as vibrations, which cause mixing, can lead to an explosive separation of the liquid from the vapour phase, which is called boiling delay. To avoid this, so-called boiling stones made of clay or pumice are added to chemical liquids that tend to boil. These are not attacked by the chemical, but their porous structure facilitates the formation of small bubbles so that boiling delay does not occur.
See also: evaporation, gasification, evaporation, transpiration, Pictet-Trouton rule
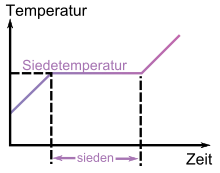
Temperature change with time when heating a pure liquid substance
Boiling Point Curve
All temperature-pressure-value pairs at the gas-liquid phase boundary in a phase diagram together result in the boiling point curve, whereby a thermodynamic equilibrium prevails on it. The boiling point curve is often referred to as the boiling curve, boiling line, boiling pressure curve or boiling point curve. This curve is bounded by two points:
- Triple point Pt: If the pressure-temperature value pair is lower than the triple temperature or the triple pressure, only a transition between solid and gaseous state, i.e. sublimation or resublimation, is possible.
- Critical point Pc: If the pair of pressure-temperature values is higher than the critical temperature or the critical pressure, there is no longer any difference between the density of the liquid and that of the gaseous state, which is why they are no longer separated by a phase boundary line and the substance is therefore called a supercritical fluid in this state.
The equilibrium of the boiling point curve is a dynamic equilibrium. From a liquid, particles constantly pass into the gas phase - they evaporate. On the other hand, these particles also re-enter the liquid phase - they condense. The numerical ratio of the particles leaving the liquid phase and the particles re-entering it depends on both the temperature and the pressure: The higher the temperature, the more particles evaporate due to their higher velocity (see Maxwell-Boltzmann distribution). The more particles evaporate, the higher the vapor pressure, and the more particles condense again. Equilibrium is reached when as many particles pass into the gas phase as return to the liquid phase. Since the gas phase is saturated in this state, this is also referred to as saturation vapour pressure. The thermodynamic law from which the boiling point curve is quantitatively derived is known as the Clausius-Clapeyron equation. For water, this relationship between saturation vapour pressure and saturation temperature can also be determined using the approximate equations of the Magnus formula type.
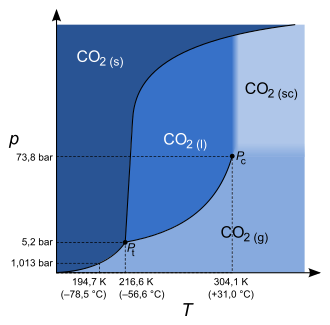
Phase diagram of carbon dioxide (not to scale)
Search within the encyclopedia