Vaccination
![]()
The title of this article is ambiguous. For other meanings, see Vaccination (disambiguation).
A vaccination, also called protective vaccination, vaccination (older vaccination) or vaccination (originally the infection with cowpox material; from Latin vacca 'cow'), is the administration of a vaccine with the aim of protecting against a (transmissible) disease. It serves to activate the immune system against specific substances. Vaccines were developed as a preventive measure against infectious diseases. Later, cancer vaccines were also developed for cancer immunotherapies.
A preventive vaccination against an infectious disease is based on a specific, active immunisation against the pathogen and is therefore also called active vaccination or active protective vaccination. The aim of active vaccination is to enable the body's own immune system to react to an infection with the pathogen so quickly and effectively that no or only a weakened infectious disease results. A distinction is made between live vaccines and inactivated vaccines; toxoid vaccines also belong to the latter. In contrast, passive vaccination (also known as curative vaccination) is merely passive immunisation through the administration of antibodies.
Vaccines are currently available against a wide range of viral and bacterial infectious diseases. Further vaccines against some significant infectious diseases and against chronic infections that promote cancer are currently under development.
For active immunization against the novel coronavirus SARS-CoV-2, an RNA vaccine was approved for the first time by a government regulatory agency in 2020.
Vaccination of an infant
.webm.jpg)
Play media file Video: What helps against viruses? (Active and passive vaccination)
Mode of action and efficacy
Administration of vaccinations
Depending on the vaccine and the type of immunisation (passive or active immunisation), different application methods are used: Active vaccinations are administered parenterally ("bypassing the gastrointestinal tract") with a syringe. A distinction is made between intradermal ("into the skin"), subcutaneous ("under the skin") or intramuscular ("into the muscle") injections. Intradermal vaccination can also be carried out with a lancet or a vaccination gun. For a few immunizations, the vaccine has been or will be administered orally (into the mouth, "swallow vaccination") or nasally (into the nose), including skin patches on an experimental basis. However, most active vaccines are administered intramuscularly into the upper arm (deltoid muscle). In children, injection into the thigh (vastus lateralis muscle) is also allowed; in young children, there are fewer local reactions after certain vaccines if they are given into the thigh. Injection of active vaccines into the medial gluteus medius muscle is considered obsolete due to lower efficacy and more frequent complications, according to the Standing Committee on Vaccination (STIKO). Passive immunizations, on the other hand, are often administered into the gluteal muscle. Powder injection is a vaccination method under development in which the solid vaccine is shot into the epidermis at high velocity.
Active vaccination
Active protective vaccination causes the body of the vaccinated person to produce its own protective substances against certain diseases. His immune system is stimulated to form a pathogen-specific immune competence without having to undergo the infectious disease itself. Live or dead vaccines are used for this purpose. A live vaccine contains attenuated pathogens that are still capable of reproducing and do not trigger the disease in the immunocompetent vaccinee. A dead vaccine, on the other hand, contains killed pathogens or only fragments of the pathogen.
Once the vaccine has entered the body, the proteins and/or sugar molecules of the vaccine are recognised as (foreign) antigens by immunocompetent white blood cells circulating in the blood and/or tissue. This is followed by the primary immune response through pathogen-specific imprinting of immunocompetent lymphocytes in the form of long-lived memory cells. The decisive factor for protection in the event of a subsequent infection is that, for the body, the antigens of the vaccine are largely similar to those of the pathogen causing the infectious disease.
If an infection occurs, the memory cells on the invaded pathogen recognize the antigens of the vaccine received earlier and cause lymphocytes to differentiate into short-lived plasma cells on the one hand, which produce antibodies, and T lymphocytes and NK cells on the other, which represent the cellular defense. The vaccination is therefore intended to induce immunity against the pathogen, so that in the event of infection, the specific and rapid immune response prevents the infectious disease.
Toxoid vaccines, which contain only the biologically inactive component (toxoid) of the toxin of a pathogen (e.g. the tetanus toxoid), also belong to the category of dead vaccines. They do not reduce the multiplication of the pathogens in the body. In the case of transmissible infections, they do not break the chain of infection, but prevent the infectious disease in the vaccinated persons, insofar as the toxins of the pathogens do not take effect in them.
Different live vaccines can be administered either simultaneously or at least four weeks apart. There are no necessary intervals for dead vaccines among themselves or in combination with live vaccines. Nevertheless, parallel administration of dead and live vaccines is preferred.
A sustainable protection builds up after a few days with live vaccines. With inactivated vaccines, repeated immunisations must be given (basic protection), followed by booster vaccinations.
A form of therapy that is similar to the principle of active immunisation, but which is not a vaccination, is hyposensitisation. It is used, for example, for hay fever or allergies to house dust mites and insects.
Passive vaccination
If a person is in danger of suffering an infectious disease because he or she has had contact with the pathogen in question without already being protected against it or being able to protect himself or herself through silent celebration or vaccination, or if the protection is to be supplemented by the person's own immune system, passive vaccination (if necessary simultaneous vaccination - see below) is indicated: Here, the recipient is injected with immune serum containing high concentrations of antibodies against the pathogen. In this way, the patient's own immune system does not produce any antibodies itself, but remains "passive". Today, monoclonal human antibodies or homologous antibodies produced by genetic engineering from cell cultures are preferred for this purpose, or, if such antibodies are not available, extracts from the blood (convalescent serum) of people who have (unintentionally) experienced the infectious disease in question, or from the blood of animals, or heterologous (foreign) antibodies that have been specifically infected with the pathogen. Passive immunisation is therefore an emergency measure in the sense of post-exposure prophylaxis. Examples of this are injuries with contamination of the wound (suspected infection with tetanus), bites by or mucosal contact with certain wild animals (suspected rabies) or contact of medical personnel with blood from patients who are carriers of the pathogens of hepatitis B (especially after needlestick injuries).
The advantage of immune sera is the faster onset of protection: the antibodies do not have to be formed within one to two weeks, but are available immediately after the injection of the immune serum. The disadvantage is that the protection only lasts a few weeks; after that, the administered antibodies are broken down by the recipient, and his organism is again at risk from a new infection with the same pathogen. This is due to the fact that the immune system is not stimulated by the administration of immune serum to form its own immune memory with regard to the pathogens via memory cells.
If the immune serum originates from an animal or human, another disadvantage is that it may contain traces of foreign protein or polysaccharides from the donor in addition to the desired antibodies. The immune system of the recipient then initiates a cascade of immunological reactions against these components, which are perceived as foreign antigens. As a result, the antibodies enriched in the vaccine serum are excreted more quickly and thus remain effective for a shorter period than desired. Repeated administration of foreign serum, especially from the same species, may also cause an adverse allergic reaction in the recipient in the form of serum sickness or allergic shock. Therefore, such immune sera are replaced by monoclonal antibodies whenever possible.
Until about 1965, for example, there were no human antibodies against tetanus, so that one had to rely on animal antibodies. The order horse, cattle, mutton had become established.
Passive immunization was introduced in 1890 by Emil von Behring when he developed a cure for diphtheria using antibodies isolated from horse blood.
An important and widespread natural form of passive immunization against infectious diseases is mother-to-child immunization.
Passive immunizations that are not directed against infectious diseases include the injection of anti-D immune serum to pregnant women if the newborn is at risk of developing haemolyticus neonatorum, and the injection of antivenin after snakebites.
Simultaneous vaccination
If a patient with possibly or known insufficient immune protection is suspected of having been infected with pathogens of a dangerous infectious disease, he or she will receive passive immunization in addition to active immunization in order to prevent a life-threatening infection. Such simultaneous active and passive immunization of a patient is called simultaneous vaccination. This involves injecting the active and passive vaccines into different parts of the body so that the injected antibodies do not immediately reabsorb the antigens (or toxins) of the protective vaccine.
Mother-Child Immunization
A temporary form of passive immunisation is mother-child immunisation, also called nest protection or surrogate immunity. Pregnant women who have developed an appropriate antibody titer after infections or vaccinations pass antibodies on to the unborn child via the placenta. After birth, the unborn child is protected to a certain extent for a few weeks to months.
Breastfeeding mothers provide the infant with secretory antibodies (sIgA), which reduce the risk of gastrointestinal diseases. However, they do not confer protection against vaccine-preventable diseases such as measles.
However, nest protection is not provided for all infectious diseases. The child vaccinations recommended in general and in Germany in particular by the Standing Commission on Vaccination should therefore be given so early that there is no gap in the pathogen defence.
Effectiveness
| Historical comparison of annual cases of infection in the United States before and after the introduction of vaccination programs (as of 1999). | ||
| Disease | before | after |
| Diphtheria | 175.885 | 1 |
| Haemophilus influenzae B | 20.000 | 54 |
| Whooping Cough | 147.271 | 6.279 |
| Measles | 503.282 | 89 |
| Mumps | 152.209 | 606 |
| Smallpox | 48.164 | 0 |
| Rubella | 47.745 | 345 |
No vaccination protects one hundred percent against the respective disease. However, vaccinations significantly reduce the probability of contracting the disease. The protective effect differs depending on the vaccination. The vaccine effectiveness is documented by many studies and, for example in Europe, vaccines are approved by the EMA (European Medicines Agency) after a benefit/risk assessment, subsequently recommended by national authorities or given on a mandatory basis.
The respective protective effect can be estimated laboratory-chemically by measuring the antibody concentration formed against the pathogen or its components, the antibody titer. The decisive factor is the efficacy within the framework of clinical studies, if possible in the form of randomised controlled trials. Here, the study participants are randomly divided into two groups. Either certain laboratory values (surrogate markers, mainly antibodies) or the frequency and severity of the infectious disease in the study group, i.e. in humans or animals that have received the vaccine to be assessed, are compared with those in the control group, i.e. in humans or animals that have not received a vaccine or have received a vaccine that is already known. Efficacy trials in which humans are deliberately infected with pathogens of serious infectious diseases are prohibited for ethical reasons, as this exposes the control group and the study group to an unjustifiable risk. Vaccines are licensed in Europe in accordance with the guidelines of the European Medicines Agency and the relevant government authorities. It requires preclinical and clinical trials and further post-marketing controls. In Germany, the Paul Ehrlich Institute reviews and monitors the approval of vaccines. Criteria and procedures are similar in other developed countries such as the USA and Canada.
There are vaccinations that so far only mitigate the course of the disease and thus only protect against the worst complications. This is particularly the case when the pathogens in question frequently change their properties through antigenic shift or antigenic drift, as is the case with the pathogens of influenza, and when the pathogens circulate in numerous antigenic subtypes, as is the case with pneumococci. Government agencies evaluate vaccines for their utility and then make an official recommendation. The recommendations have consequences under health insurance, liability and medical law.
Modern vaccines against tetanus, hepatitis, meningococcus, pneumococcus and cervical cancer contain the salt aluminium hydroxide as an effect enhancer in order to reduce the number of vaccination cycles required.
Since the introduction of vaccination in the United States, the number of annual cases of diphtheria, mumps, pertussis, and tetanus has fallen by more than 92%, while the number ofdeaths from these diseases has decreased by at least 99%. Poliovirus, measles, and rubella virus are considered eradicated in the United States, and in 1980 the World Health Organization (WHO) declared the world free of smallpox.
Conversely, according to estimates by the WHO and the Global Alliance for Vaccines and Immunization (GAVI), more than two million people died worldwide in 2002 alone from infectious diseases that could have been prevented by vaccination. Reducing these causes of death through vaccination programmes is therefore a primary goal of WHO. The success of these vaccination programmes demonstrates the effectiveness of vaccination. Most available vaccines are listed in the Recommended Vaccines section.
Weakening of the effectiveness due to interaction with painkillers
Vaccines are medicinal products and drug interactions may occur when several medicinal products are used simultaneously. They can consist of an increase or decrease in efficacy, an increase or decrease in known side effects or the occurrence of new side effects. Recently, there has been increasing evidence that certain drugs from the group of non-steroidalanti-inflammatory drugs (NSAIDs) or non-steroidal anti-inflammatory drugs such as acetylsalicylic acid (aspirin), but also other non-opioid analgesics such as paracetamol and substances derived from them, can reduce the effectiveness of vaccines. This is attributed to the fact that these drugs achieve their antipyretic and (in the case of the NSAIDs) also anti-inflammatory effect by inhibiting certain enzymes, the cyclooxygenases (COX), which is why they are also called cyclooxygenase inhibitors. However, COX, which is involved in prostaglandin synthesis, also plays an important role in immune defence. Blocking the enzyme apparently has the side effect of decreasing the production of protective antibodies after vaccination by impairing the terminal differentiation of B cells into antibody-producing plasma cells. Therefore, it is recommended to avoid COX-inhibiting drugs for some time before and after vaccination.
Refresh
After basic immunisation, regular booster vaccinations are necessary for the long-term maintenance of immunity against most pathogens. The booster vaccination (also called booster vaccination or repeat vaccination) differs from the basic immunization in that it leads to sufficient clinical protection of the patient again within a short period of time even with the single administration of a lower vaccine dose. Recommendations for the timing of such booster vaccinations are based on observations of the use of individual vaccines and depend on both the type of pathogen and the vaccine: some vaccinations can confer protection that is very likely to last a lifetime even without a booster. For example, individuals vaccinated against smallpox have been shown to retain immunity for up to 88 years after vaccination, which was comparable to having survived the disease. In persons who had been vaccinated against measles, mumps and rubella, sufficiently high antibody titres were found for the most part even 20 years after vaccination. The same appears to be true for vaccinations against hepatitis A. Other vaccines, such as the pertussis vaccine, require a booster every ten years according to current recommendations, as antibody levels decline after four to twelve years. However, natural immunity from infection with pertussis also wears off after four to 20 years. In contrast, vaccination against influenza with the current vaccines must be repeated annually.
The booster vaccination should not be confused with the necessary partial vaccinations that are required for a completed basic immunisation, depending on the vaccine. These come into play, for example, in the "2+1" scheme or "3+1" scheme. In the 3+1 scheme, the partial vaccinations required for basic immunisation take place three times at intervals of four weeks, followed six months later by the booster vaccination. In the 2+1 scheme, the interval between the two basic vaccinations is eight weeks, and the booster vaccination is given six months later.
Side effects
→ Main article: Reactogenicity
The side effects of today's officially recommended vaccinations are usually so minor that they are not perceived or are not perceived as significant. A distinction is made between vaccine reaction and vaccine complication. Vaccine reactogenicity refers to the extent and clinical significance of the expected vaccine reaction.
When evaluating reactions after vaccination, it must always be kept in mind that vaccinations are carried out in healthy people and that a subsequent illness is strongly felt. The expectation of severe side effects can lead to increased self-monitoring (nocebo effect). As a consequence, randomly occurring disturbances of well-being, which would normally not be noticed at all, can suddenly be consciously perceived and erroneously blamed on the vaccination.
A so-called vaccination reaction is a short-term and temporary local and general reaction. These can occur as temporary, mild side effects such as pain, tension and swelling at the injection site, fatigue or headache and pain in the limbs. In double-blind trials without exposure to pathogens, where half of the volunteers are injected with the vaccine and the other half with saline or an adjuvant containing aluminium hydroxide, both groups report quantitatively and qualitatively similar side effects for most officially recommended vaccines: e.g. dizziness, headache, feeling of weakness, muscle pain (see also nocebo effect).
| Comparison of complications of disease and after vaccination against measles, mumps and rubella (MMR). | ||
| Symptom/disease | Complication rate with | complication rates after |
| Measles | MMR | |
| Exanthem | 98 % | 5 %, attenuated |
| Fever | 98 %, mostly high | 3 to 5 %, very rarely high |
| Febrile convulsions | 7 to 8 % | ≤ 1 % |
| thrombocytopaenia | 1/3000 | 1/30,000 to 1/50,000 |
| Encephalitis | 1/1000 to 1/10,000 | 0 |
| Lethality | 1/1000 to 1/20,000 | 0 |
| Mumps | MMR | |
| salivitis | 98 % | 0,5 % |
| Pancreatitis | 2 to 5 % | 0,5 % |
| Inflammation of the testicles in adolescents | 20 to 50 % | 1/1.000.000 |
| Meningitis | ~ 15 % | 1/1.000.000 |
| Numbness | 1/20.000 | 0 |
| Rubella | MMR | |
| Joint complaints in | 40 to 70 %, persistent | 1/10,000, mostly short |
| Encephalitis | 1/6000 | 0 |
| thrombocytopaenia | 1/3000 | 1/30,000 to 1/50,000 |
| Rubella embryofetopathy in infection | > 60 % | 0 |
A vaccine complication is a complication after a vaccination that goes beyond the usual extent of a vaccine reaction. In rare cases, live vaccines can lead to an outbreak of the disease that was vaccinated against. For example, five percent of those vaccinated against measles experience non-infectious so-called "vaccine measles" that resolve after 1-3 days. The side effects of the vaccination then include the symptoms of the disease, for example, mild rash or fever; however, they are usually milder than the "natural" infection. In very rare cases, allergic-anaphylactic shock may occur as a reaction to the ingredients of a vaccine dose. This has been observed in 33 cases (often with pre-existing conditions such as atopy or asthma) in 25.2 million vaccine doses administered, none of which were fatal. In addition to the active ingredient itself, additives such as aluminium compounds, mercury compounds (thiomersal), formaldehyde and antibiotics or substances from the manufacture of the active ingredient such as hen's egg white can trigger such a reaction. Doctors must provide adequate information about this risk, as well as the risk of vaccination, prior to vaccination. Those who vaccinate must be prepared, through practice and appropriate equipment, to handle possible life-threatening allergic reactions to vaccination.
Vaccines are not known to cause any long-term side effects or long-term damage (damage that only occurs years later). This is because the components of a vaccination are rapidly broken down in the body. As a result, most side effects are mainly observed within hours or days. Long-term effects refer to very rare side effects that can only be statistically recorded after many years.
Since 1 January 2001, physicians in Germany have been subject to the "obligation to report suspected adverse health effects beyond the usual extent of a vaccination reaction" as laid down in the Infection Protection Act (IfSG). According to § 6 para. 1, no. 3 IfSG, physicians are obliged to report to the public health department if symptoms occurring after a vaccination that go beyond a vaccination reaction could be causally related to the vaccination. This reporting system is a so-called spontaneous detection system to identify early risk signals of vaccination side effects that were not recorded during the licensing process. By 31 December 2003, 3328 cases of possible vaccine adverse events had been recorded in all age groups (in three years, for approximately 30 million vaccine doses/year). Of these, four percent suffered permanent damage and 1.6% died (mainly documented coincidences). In the majority of suspected cases reported to the Paul Ehrlich Institute (PEI), the causal relationship between vaccination and disease was assessed as unlikely. In the other cases, the causal relationship with vaccination could not be assessed due to a lack of valid scientific data. An association between vaccination and reaction is considered certain in only 0.2% of all IfSG notifications. In Germany, there is an entitlement to benefits from the pension offices if a health disorder is possibly due to a publicly recommended vaccination. However, the patient does not have to prove that the vaccination was the cause of his illness.
Comparing the numbers of possible vaccination reactions with the vaccinations carried out in the same period results in a very low risk, for example 250 IfSG reports on possible reactions of about six to eight million vaccination doses to the MMR vaccine in the same period. However, despite the legal obligation to report, the rate of reporting depends on the motivation and ability of physicians. Therefore, spontaneous recording alone is not suitable for estimating the frequency of vaccination side effects. This is where actively recording pharmacovigilance systems and studies geared to the respective vaccination complication come in.
In general, vaccines have an excellent safety profile.
"False" contraindications
Often indicated vaccinations are omitted (or postponed indefinitely and eventually forgotten) because certain circumstances are mistakenly regarded as obstacles to vaccination. According to the Robert Koch Institute, these are in particular:
- banal infections, even if accompanied by a slight fever (up to 38.5 °C),
- History of febrile convulsions in the patient,
- Antibiotic use,
- immunodeficiency (except in the case of certain live vaccines; in general, however, immunocompromised persons are particularly dependent on vaccination protection) and
- chronic diseases (on the contrary, vaccination is especially important for the chronically ill).
Economic savings
The cost-benefit ratio of vaccinations has been determined on the basis of model calculations. For example, a calculation in Austria in 2003 showed that each measles vaccination results in an economic saving of approx. 600 euros due to the measles disease avoided as a result. The costs of treating the side effects associated with a measles infection were taken into account.
The considerable economic benefit of vaccinations could also be shown for pneumococcal vaccination by the analysis of Silvia Evers and colleagues of the University of Maastrich in 2007. The upper limit was 50,000 euros per quality-adjusted life year (QALY; in short, 1 QALY corresponds to one year of life spent in perfect health). Measures that cost less than 50,000 euros per QALY are considered cost-effective by health economists. Evers' analysis covered ten European countries. The resulting cost-benefit ratios ranged from 9239 (Denmark) to 23,657 euros per QALY (Sweden). Germany ranks in the middle of these calculations with 17,093 euros per QALY.
Other international studies (e.g. on polio, whooping cough or hepatitis B) on the economic benefits have shown that vaccination can lead to considerable savings.
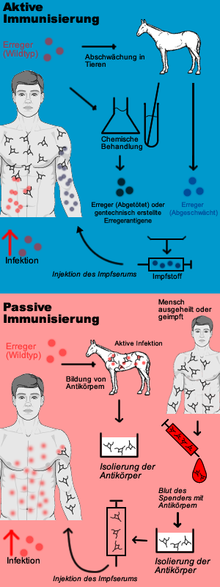
Scheme of active/passive immunization
Mode of action on the spread of infectious diseases
| Minimum proportion of immunised persons required for herd effect in | |||
| Disease | Transmission path | R0 | Minimum percentage immunised |
| Measles | Droplet infection | 12–18 | 83–94 % |
| Mumps | Droplet infection | 4–7 | 75–86 % |
| Polio | fecal-oral infection | 5–7 | 80–86 % |
| Rubella | Droplet infection | 5–7 | 80–85 % |
| Smallpox | Droplet infection | 6–7 | 83–85 % |
| The basic reproduction number R0 indicates how many more people an infected person will infect | |||
Vaccination also affects the spread of infectious diseases in a population. The specialty of mathematical modeling in epidemiology studies the epidemiological behavior of infectious diseases and can calculate the effects of vaccination programs. Under certain conditions, high vaccination coverage rates in a population can induce herd immunity (collective immunity) in addition to immunity of the vaccinated, which also serves to protect the unvaccinated from disease because the high proportion of immunized individuals limits the circulation of the pathogen within the population. The herd effect reduces the risk of exposure of the unvaccinated, such as infants, the elderly or immunodeficient patients, to pathogens to which they themselves are not immune.
If, in the case of a local outbreak of an infectious disease, an attempt is made to build up herd immunity by means of a rapid vaccination campaign, this is also referred to as bar vaccination.
According to the Robert Koch Institute, vaccinations are among the "most important and effective preventive measures available in medicine". Since the middle of the 20th century, comprehensive vaccination programmes have led to a massive reduction in various infectious diseases or even to their regional or - as in the case of smallpox - global eradication. They also contribute to a reduction in mortality among children, as studies in the Netherlands have shown. The U.S. Centers for Disease Control and Prevention (CDC) ranks vaccination among the top ten achievements of medicine and public health. Vaccination is thus the most significant part of the disposition prophylaxis within the general protection against infection.
As late as the twentieth century, an estimated 375 million deaths occurred worldwide until smallpox was eradicated in 1978; other infectious diseases now virtually unavoidable through vaccination still claimed 39 million lives in the United States in the twentieth century. It is estimated that 1.5 million children (three per minute) continue to die annually from vaccine-preventable infections.
Questions and Answers
Q: What is a vaccination?
A: A vaccination is a treatment which makes the body stronger against an infection by introducing something to the immune system that looks very similar to a particular virus or bacteria.
Q: What does the immune system do?
A: The immune system fights infections using millions of cells, including T cells and B cells.
Q: How does the adaptive immune system work?
A: The adaptive immune system is much stronger when fighting a disease that it has already fought against before.
Q: How does vaccination help strengthen the body's immunity?
A: Vaccination involves showing the immune system something which looks very similar to a particular virus or bacteria, which helps the immune system be stronger when it is fighting against the real infection.
Q: What are T cells and B cells?
A: T cells and B cells are two types of cells that make up part of the millions upon millions of cells in our bodies' immune systems.
Q: Does everyone need vaccinations?
A: Vaccinations are recommended for many people depending on their age, health status, lifestyle, and other factors; however, not everyone needs every type of vaccine. It's important to speak with your doctor about what vaccines you may need based on your individual circumstances.
Q: Are there any risks associated with vaccinations?
A: While most people experience no side effects from vaccines, some people may experience mild reactions such as soreness at injection site or fever after receiving certain vaccines. In rare cases more serious side effects can occur; however these are usually temporary and treatable.
Search within the encyclopedia